Figure 3.
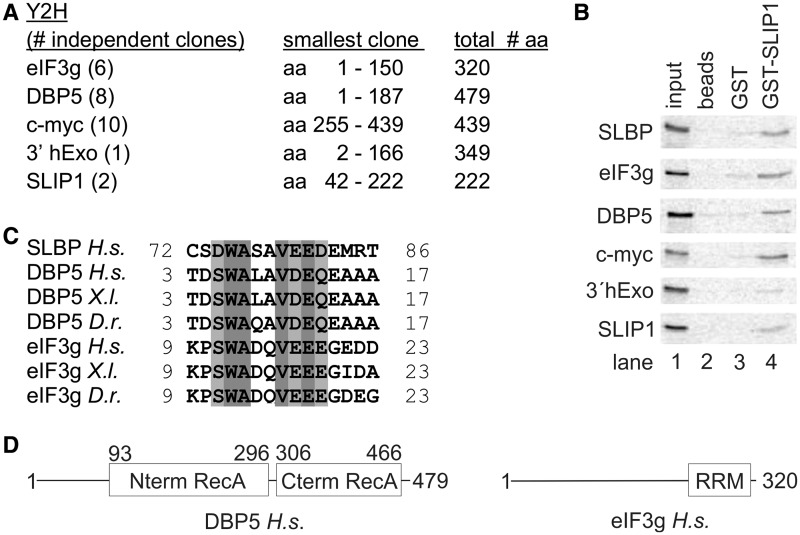
SBMs are present in eIF3g and DBP5. (A) A two-hybrid screen was carried out using SLIP1 as bait. Over 200 positive clones were characterized by isolating the insert by polymerase chain reaction and digesting the fragment with AluI to identify clones that contained overlapping inserts. Over 120 of these clones encoded DBP5, eIF3g or myc. A number of clones of different sizes from each were sequenced (indicated in parentheses). The length of the protein in amino acids is shown, together with the smallest positive clone isolated. Smaller numbers of clones for several other proteins were obtained. (B) To validate the two-hybrid results, each of the proteins was labeled with 35S-methionine by in vitro translation and analyzed in a pull-down assay as in Figure 2D. (C) Alignment of the SBM of H.s. SLBP with the putative SBMs we identified in eIF3g and DBP5 in several vertebrates. (D) Schematic representation of the domain organization of human DBP5 and eIF3g. The folded domains of DBP5 and eIF3g are boxed and labeled. In each protein, the putative SBMs reside in regions of low complexity that are predicted to be unstructured.