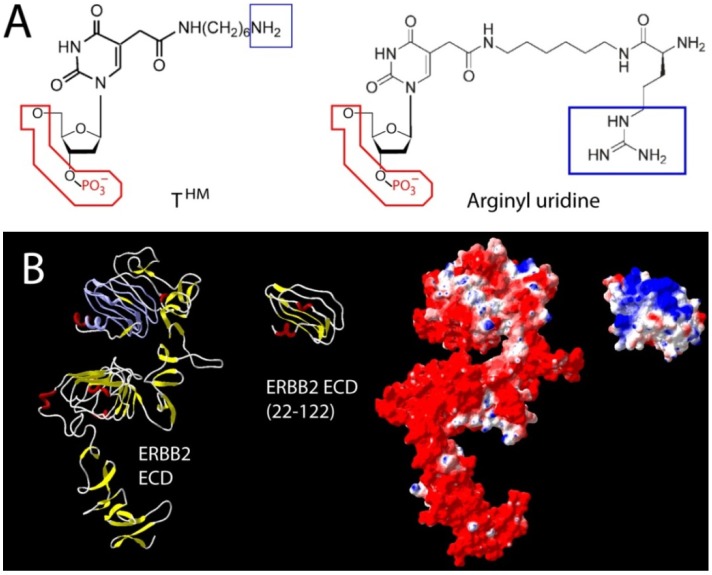
Figure 3.
Two different approaches used to counteract charge repulsion of nucleic acid aptamers. (A) THM (5-N-(6-aminohexyl) carbamoylmethy-2-O-deoxyuridine) and arginyl uridine represent two modified bases that can be enzymatically incorporated. This approach yielded aptamers against Silalyllactose [66] (THM), or glutamic acid [80] and carboxy glutamic acid [81] respectively (arginyl-uridine); (B) Aptamer selection against the extracellular domains of ERBB2 (HER2) has been challenging due to its uniformly distributed negative surface charge (red in surface representation). In the absence of its extreme N- and C-terminal capping regions, a segment (22–122) of domain one provided suitable bait for the selection of a nanomolar RNA aptamer. Once selected, the final 2' Fluoro RNA aptamer retains its high affinity in vitro and in vivo in the presence of surrounding negative surface charges [79]. Truncations and surface charge calculations of the reference structure for ERBB2 [82] were generated in Swiss PDB viewer using default parameters.