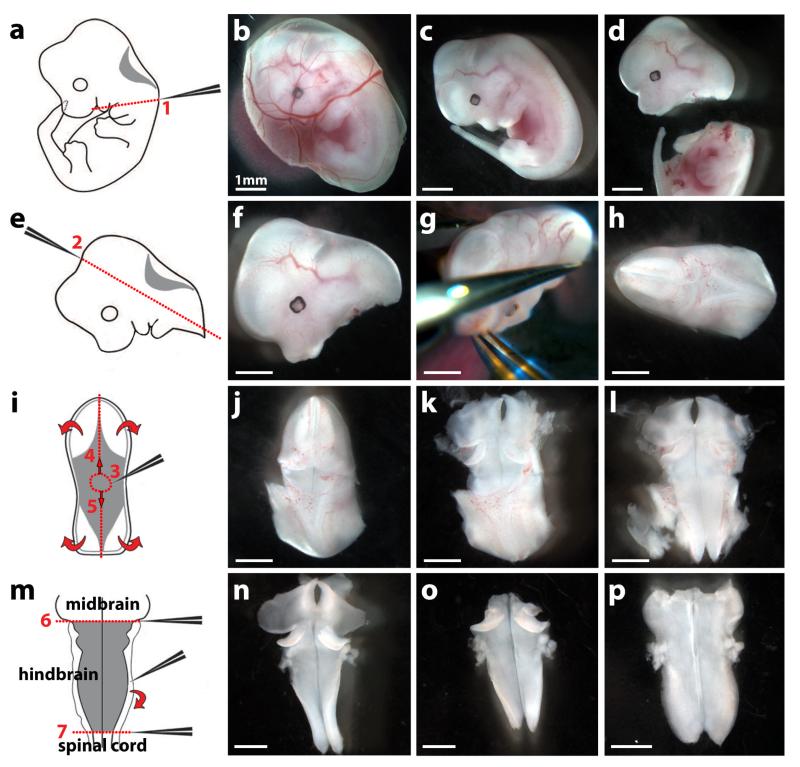
Fig. 4. Dissection of an E12.5 mouse embryo hindbrain.
The left hand side of the figure shows schematics and the right hand side dissected tissues.
(a-d) Remove the embryo and its yolk sac from the uterus with forceps (b); separate the embryo from the yolk sac (c); remove the head from the body with forceps by applying pressure along line 1 (a,d).
(e-h) Position the head (f) in a convenient orientation (g) and apply pressure along line 2 with forceps (e,g) to obtain the hindbrain with surrounding mesenchymal tissue (h).
(i-l) Position the hindbrain with surrounding mesenchymal tissue in a convenient orientation (j) and make a hole within the roofplate by inserting the forceps in position 3 (i,j). Use forceps to tear the roofplate along the dotted lines 4 and 5 (i) to expose the hindbrain (k) and allow it to unfurl (curved arrows in i; l).
(m) Tease away the pial membranes and surrounding mesenchyme from the neural tissue using forceps (curved arrow in m,n). Squeeze with forceps along the dotted lines 6 and 7 to remove midbrain and spinal cord tissue, respectively (m,o). The hindbrain now unfurls completely, ready for formaldehyde fixation; it can be easily visualised after carefully covering it with a glass coverslip (p).
Scale bars: 1 mm. All animal procedures were performed in accordance with institutional and UK Home Office guidelines. Adapted with permission from ref. 28.