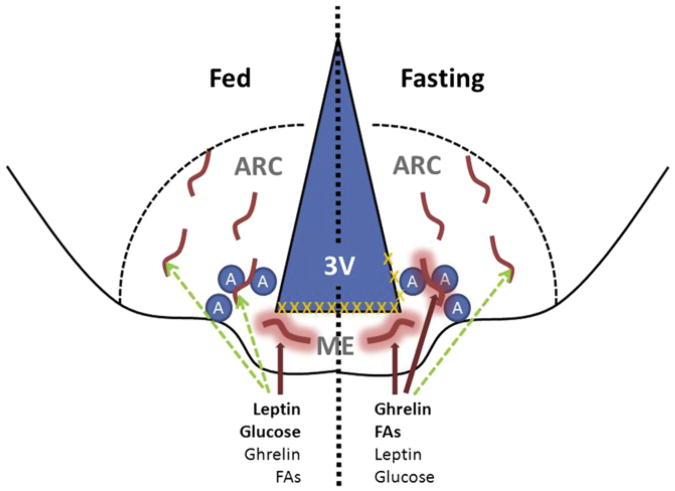
Figure 1. Model for the Fasting-Induced Control of Vascular Permeability at the ARC/ME Junction.
The arcuate nucleus of the hypothalamus (ARC) lies lateral to the third cerebral ventricle (3V) and immediately dorsolateral to the median eminence (ME). Orexigenic AgRP/NPY-expressing neurons (A) lie near the ME/ARC junction. Under normal fed conditions (left half of figure), the ME contains fenestrated blood vessels that permit the local diffusion of macromolecules from the circulation (thick red lines with halo), while the vessels in the ARC proper exhibit BBB properties that do not permit diffusion (no halo). Hence, peptides and metabolites that are high in the fed state (e.g., leptin and glucose) require BBB transport (dashed green lines) to access ARC cells. Tight junctions (orange Xs) lie above the ME under these conditions, preventing the diffusion of circulating factors into the 3V and CSF. During energy restriction (right half of figure), peptides such as ghrelin rise, along with substrates derived from lipolysis (e.g., fatty acids [FAs]), while leptin and glucose fall. Concomitantly, some of the vessels that lie along the ME/ARC junction become fenestrated, while barrier formation along the 3V extends dorsally. This change allows freer diffusion of circulating signals that indicate energy restriction to the ARC neurons (presumably AgRP cells) that lie at the ME/ARC junction, while preventing free access of these substances further into the brain and CSF. This focal plasticity in the BBB enhances the orexigenic/anabolic response to energy deficits.