Table 1.
Optimization of Cu/nitroxyl catalyzed aerobic oxidation of primary amine to nitrile.a
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | Cu | ligand | nitroxyl | base | yield (%)b | |
|
| ||||||
| 2a | 3a | |||||
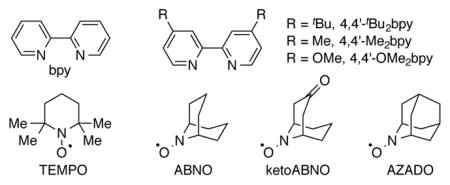
| 1 | CuOTf | bpy | TEMPO | NMI | 25 | 50 |
| 2 | CuI | bpy | TEMPO | NMI | 27 | 34 |
| 3 | CuI | bpy | ABNO | NMI | 78 | 22 |
| 4 | CuI | bpy | AZADO | NMI | 77 | 23 |
| 5 | CuI | bpy | ketoABNO | NMI | 53 | 46 |
| 6 | CuI | 4,4′-tBu2bpy | ABNO | NMI | 87 | 13 |
| 7 | CuI | 4,4′-Me2bpy | ABNO | NMI | 80 | 14 |
| 8 | CuI | 4,4′-OMe2bpy | ABNO | NMI | 74 | 26 |
| 9 | CuI | 4,4′-tBu2bpy | ABNO | DMAP | 90 | 4 |
| 10c | CuI | 4,4′-tBu2bpy | ABNO | DMAP | 75 | 24 |
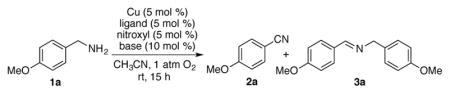
Conditions: 1a (0.5 mmol), Cu, ligand, nitroxyl, and base in CH3CN (2.0 mL) under O2 balloon at room temperature for 15 h.
Yield determined by 1H NMR (internal standard: 1,1,2,2-tetrachloroethane).
Carried out under air.