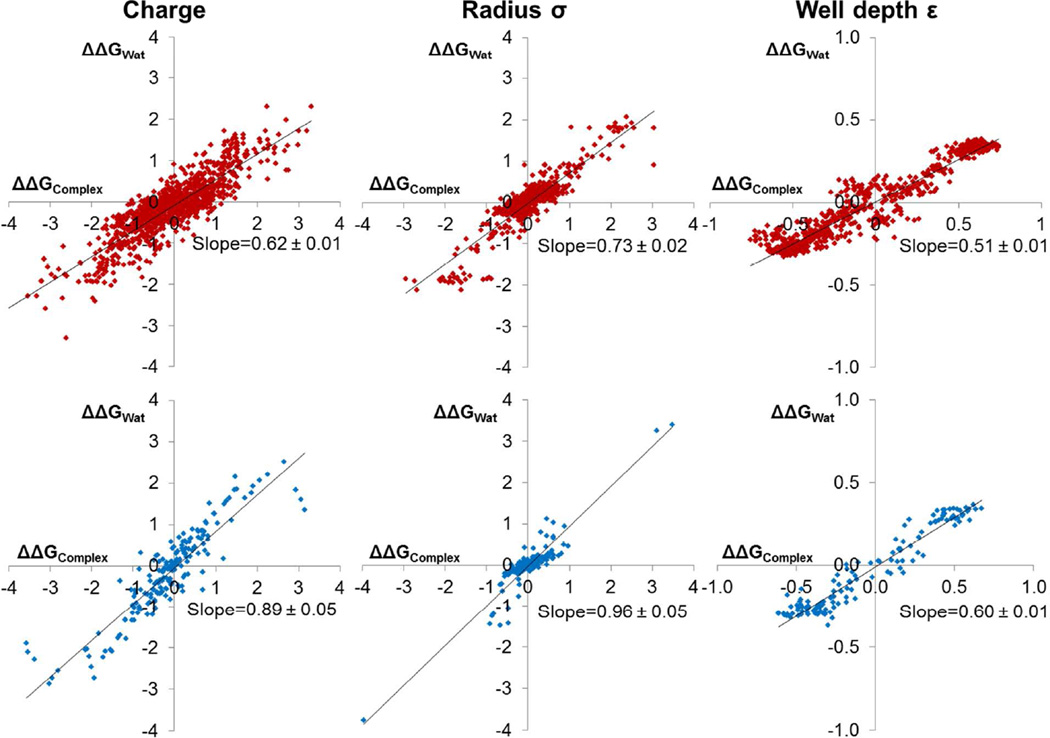
Figure 8. Cancellation of effects in solvent and in the protein buffers the overall ΔGBind to changes in parameters.
In CCP (top, red), ΔΔGWat buffers 35–50% of the change in ΔΔGComplex (despite slopes of 0.6–0.7), while in Gyrase (bottom, blue) this buffering is even stronger. Best fit lines are shown in black. Slope uncertainties represent one standard deviation from bootstrapping calculations.