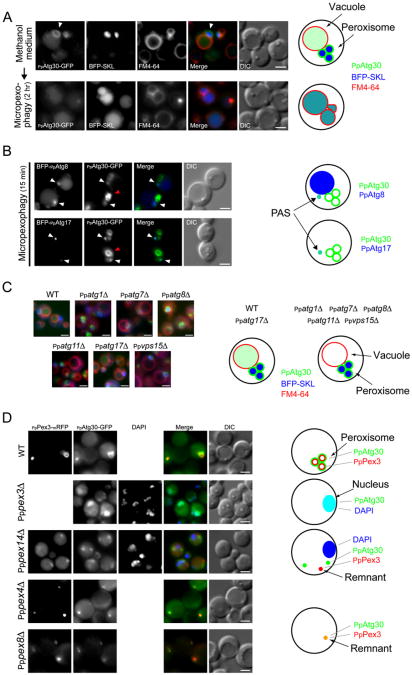
Figure 2. PpAtg30 Colocalizes with Peroxisomes, and with the PAS, as well as with Vacuoles, during Pexophagy.
(A) PpAtg30 colocalizes with the peroxisomes. Ppatg30Δ cells coexpressing PpAtg30-GFP and BFP-SKL (SJCF385) were grown overnight in methanol medium with FM4-64, shifted to glucose medium for 2 hr, and examined by fluorescence microscopy. Arrowhead indicates peroxisomes.
(B) PpAtg30 colocalizes with PpAtg8 and PpAtg17. Ppatg8Δ cells coexpressing PpAtg30-GFP and BFP-PpAtg8 (SJCF764), and wild-type cells coexpressing PpAtg30-GFP and BFP-PpAtg17 (SJCF409), were grown overnight in methanol medium, adapted to glucose medium for 15 min, and examined by fluorescence microscopy. White arrow, colocalization of PpAtg30-GFP with BFP-PpAtg8 and/or BFP-PpAtg17, probably at the PAS; red arrow, PpAtg30 around the peroxisome.
(C) Fluorescence microscopy of FM4-64-stained wild-type (SJCF393), Ppatg1Δ (SJCF624), Ppatg7Δ (SJCF387), Ppatg8Δ (SJCF389), Ppatg11Δ (SJCF623), Ppatg17Δ (SJCF390), and Ppvps15Δ (SJCF391) cells expressing BFP-SKL and PpAtg30-GFP. Cells were grown in methanol medium for 6 hr.
(D) Fluorescence and DIC microscopy of wild-type (SJCF768), Pppex14Δ (SJCF587), Pppex4Δ (SJCF769), and Pppex8Δ (SJCF770) cells coexpressing PpPex3-mRFP and PpAtg30-GFP; and Pppex3Δ (SJCF330) cells expressing PpAtg30-GFP. Nuclei were stained with DAPI. Cells were grown in methanol medium for 6 hr. PpAtg30 was expressed from its endogenous promoter in (A), (B), (C), and (D). Bars = 2 μm.