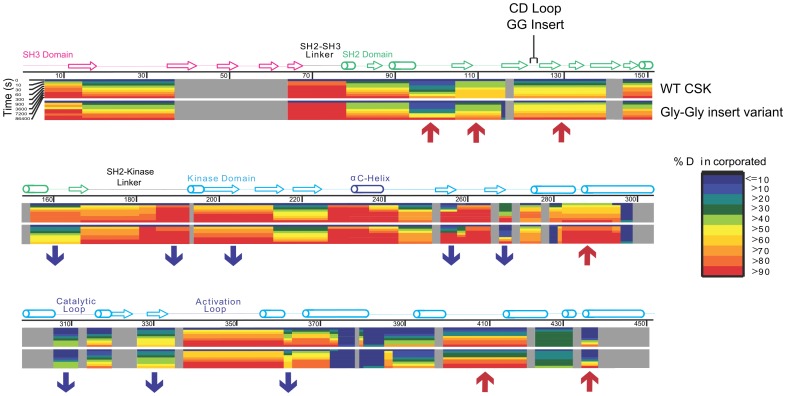
Figure 3. Heatmap schematic of % Hydrogen-Deuterium exchange showing relative amide proton protection from D2O solvent in full length variant and wild type Csk.
Color-coded blocks show levels of deuteration for peptide probes in wild type Csk (top row) and the variant (bottom row) as indicated on the right. Regions of secondary structure motifs and domains identified from the crystal structure are shown above the sequence. The figure was generated by mapping all reliably-identified peptides onto Csk's wild type sequence then overlaying the resulting blocks with exactly matching peptides that have the same mass, charge and retention times in LC-MS analysis for both proteins. The level of protection is indicated by coloring each peptide at any given time-point based on the maximum percentage of deuterons incorporated according to the key on the right. Observed differences are indicated with red upward arrows for segments that show relative deprotection while blue downward arrows indicate relative protection in the variant with respect to wild type. A difference greater or equal to 10% in protection is considered significant if observed in at least two time points. Gray blocks indicate absence of reliable probes for that part of the sequence. Peptide identification and analysis were performed after combining two independent pools of generated peptides for each of the wild type and variant Csk for verification.