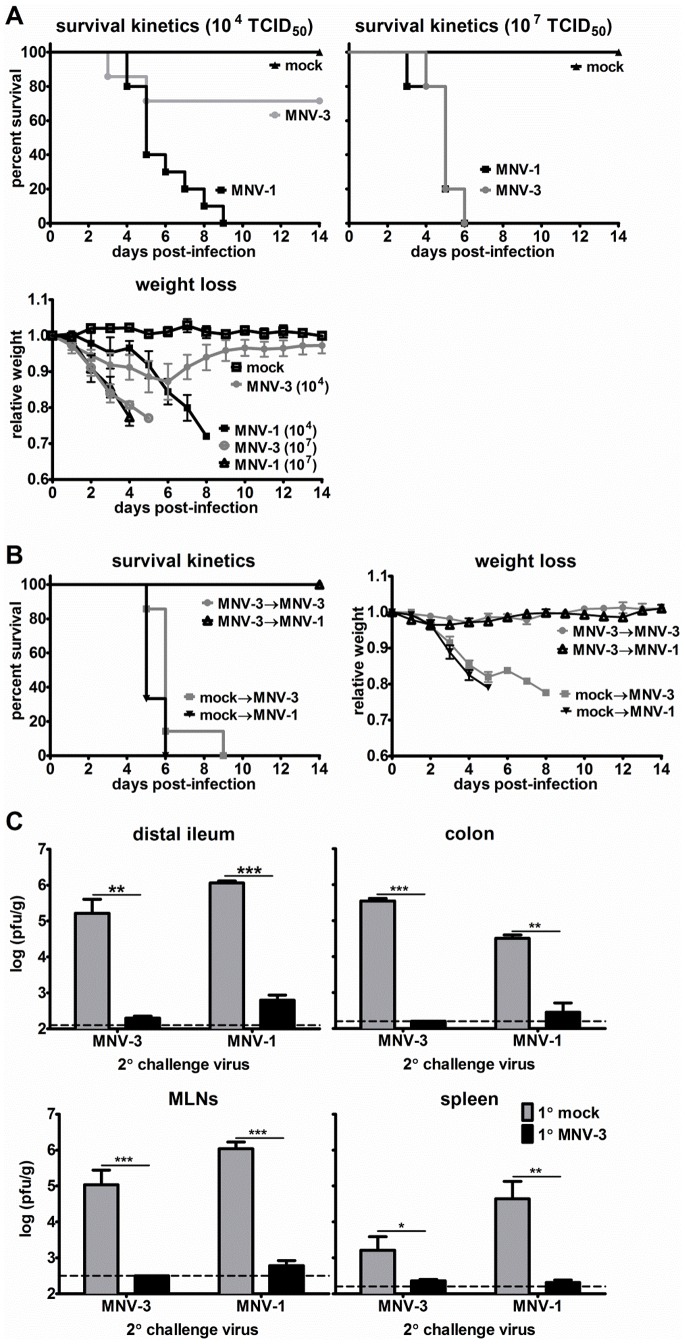
Figure 2. MNV-3 elicits protection from severe disease in the absence of type I interferon signaling.
A) Groups of mice lacking the type I interferon receptor (IFNAR−/−; a minimum of 5 mice per condition) were inoculated with mock inoculum, 104 or 107 TCID50 units of MNV-1 or MNV-3 by the peroral route. The percentage of mice surviving infection was calculated daily. All surviving mice were weighed daily and the weights compared to day 0 weights to calculate a relative weight. B) Groups of IFNAR−/− mice (n = 3–5) were inoculated with either mock inoculum or 5×103 TCID50 units of MNV-3. Six weeks later, mice were challenged with 107 TCID50 units of MNV-3 or 106 TCID50 units of MNV-1 and monitored for survival and weight loss, as described above. C) Groups of IFNAR−/− mice (n = 3) were inoculated with mock inoculum (1° mock; grey bars) or 5×103 TCID50 units of MNV-3 (1° MNV-3; black bars) by the peroral route. Six weeks later, mice were infected with 107 TCID50 units of MNV-1 or MNV-3 (2° challenge virus displayed on the x-axis). One day following 2° challenge, animals were perfused, the indicated organs harvested, and viral burden determined by plaque assay. Limits of detection are indicated by dashed lines. MLNs = mesenteric lymph nodes. Groups of mice receiving mock versus MNV-3-1° infection and the same 2° challenge virus were compared for statistical purposes.