Abstract
Polynitroxylated-pegylated hemoglobin (PNPH), a bovine hemoglobin decorated with nitroxide and polyethylene glycol moieties, showed neuroprotection vs. lactated Ringer's (LR) in experimental traumatic brain injury plus hemorrhagic shock (TBI+HS). Hypothesis: Resuscitation with PNPH will reduce intracranial pressure (ICP) and brain edema and improve cerebral perfusion pressure (CPP) vs. LR in experimental TBI+HS. C57/BL6 mice (n=20) underwent controlled cortical impact followed by severe HS to mean arterial pressure (MAP) of 25 to 27 mm Hg for 35 minutes. Mice (n=10/group) were then resuscitated with a 20 mL/kg bolus of 4% PNPH or LR followed by 10 mL/kg boluses targeting MAP>70 mm Hg for 90 minutes. Shed blood was then reinfused. Intracranial pressure was monitored. Mice were killed and %brain water (%BW) was measured (wet/dry weight). Mice resuscitated with PNPH vs. LR required less fluid (26.0±0.0 vs. 167.0±10.7 mL/kg, P<0.001) and had a higher MAP (79.4±0.40 vs. 59.7±0.83 mm Hg, P<0.001). The PNPH-treated mice required only 20 mL/kg while LR-resuscitated mice required multiple boluses. The PNPH-treated mice had a lower peak ICP (14.5±0.97 vs. 19.7±1.12 mm Hg, P=0.002), higher CPP during resuscitation (69.2±0.46 vs. 45.5±0.68 mm Hg, P<0.001), and lower %BW vs. LR (80.3±0.12 vs. 80.9±0.12%, P=0.003). After TBI+HS, resuscitation with PNPH lowers fluid requirements, improves ICP and CPP, and reduces brain edema vs. LR, supporting its development.
Keywords: blood substitute, cerebral edema, hemoglobin blood oxygen carrier, intracranial pressure, nitroxide, resuscitation
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in civilian and military settings and is often accompanied by secondary insults such as hemorrhage. Optimum resuscitation is critical given the severe consequences of hemorrhagic shock (HS) after TBI. Early hypotension is a strong clinical predictor of mortality after TBI, doubling mortality rates with a reduction in systolic blood pressure of as small as 10 mm Hg.1 In blast polytrauma victims in Operation Iraqi Freedom, sustained hypotension was associated with 100% mortality.2 Ischemia as a consequence of hypotension greatly worsens outcomes in TBI models.3 Hypotension leads to impaired energy metabolism,4 reduced neuroprotective gene expression,5 and increased neuronal death.6, 7, 8 After TBI, cerebral blood flow (CBF) can also be reduced.9 Animal models of TBI have revealed impaired autoregulation, exacerbating ischemic injury.10 At autopsy, most TBI patients show evidence of cerebral ischemia,11 implicating hypoperfusion in secondary injury after TBI.
Optimized resuscitation of TBI patients is critical given the consequences of hypotension, and multiple fluids have been studied. Traditional resuscitation fluids for TBI patients include lactated Ringer's (LR) or normal saline for civilians or the colloid Hextend (Hex) for military personnel; however, large volumes of these fluids are often required, potentially increasing brain edema and raising intracranial pressure (ICP).12 In prior models of TBI+HS, the use of hypertonic or colloid-based resuscitation reduced fluid requirements, brain edema, and ICP.13, 14 Clinical trials, however, failed to show benefit from resuscitation with hypertonic fluids or albumin.15, 16 Given these limitations, there has been interest in hemoglobin (Hb)-based oxygen carriers (HBOCs).
Cell-free Hb was recognized early for its potential role in transfusion medicine.17 The potential benefits of an HBOC that did not require cross matching could tolerate sterilization and had a longer shelf life were obvious. Hemoglobin outside the red blood cell, however, readily dissociates into dimers, which have a short half-life, high oxygen affinity (i.e., reduced oxygen release to tissues), are nephrotoxic, scavenge nitric oxide (NO), and cause oxidative stress.17 First-generation HBOCs focused on stabilization of the Hb molecule as a tetramer via cross linkage. This improved half-life and reduced oxygen affinity (higher p50).17 The efficacy of these crosslinked Hbs to deliver oxygen to target tissues was shown in experimental shock.18
Cell-free Hb and early generation HBOCs, however, rapidly scavenge NO in the subendothelium causing vasoconstriction. This can decrease blood flow, increase release of pro-inflammatory mediators, activate platelets, and promote thrombosis.19, 20 The NO scavenging by HBOCs may mediate many of the adverse effects seen in clinical trials, including myocardial ischemia.21 Strategies to prevent NO scavenging have focused on either increased molecular size or Hb modification. Increasing Hb size prevents extravasation to the subendothelium, reducing its interaction with NO. Modification of the Hb could also reduce NO affinity. The recombinant human Hb rHb2.0 (Baxter, Boulder, CO, USA) had a 20- to 30-fold reduced NO affinity,22 but animal models still showed myocardial injury.23 These attempts to reduce NO scavenging were unsuccessful, possibly because they fail to address the pro-oxidant effect of Hb.24
Hemoglobin is an oxygen carrier and a pro-oxidant, and participates in complex redox processes involving the heme iron center. The heme iron readily auto-oxidizes from the oxygen carrying ferrous state (Hb Fe2+) to the nonoxygen carrying methemoglobin (MetHb) state (Fe3+), and can further oxidize to the ferryl state (Fe4+), which can peroxidize lipids, degrade carbohydrates, and modify proteins.25, 26 Also, Hb oxidation can trigger the overproduction of reactive oxygen species, with tissue damage.26, 27 However, Hb, confined in red blood cells, is surrounded by enzymes to protect against the generation of reactive oxygen species, including MetHb reductase, superoxide dismutase, catalase, and glutathione peroxidase.25, 26, 28 Extracellular Hb lacks these protective enzymes; its oxidative potential is left unchecked. Earlier generation HBOCs failed to prevent the oxidative potential of the heme iron. Polynitroxylated-pegylated hemoglobin (PNPH) is a novel bovine Hb-based solution created for use in small volume resuscitation and was designed to eliminate the drawbacks to its Hb-based chemistry. It contains a modified bovine carboxy-Hb tetramer, covalently bound to nitroxide moieties and polyethylene glycol side chains. Polynitroxylation of the Hb confers antioxidant effects and prevents NO scavenging, while the polyethylene glycol side chains create a ‘hydrating shell' with significant oncotic effects. Polynitroxylated-pegylated hemoglobin, though based on an HBOC, is being developed primarily as a resuscitation fluid rather than as a blood substitute.24
We recently published a study on the use of PNPH, which showed a marked decrease in the fluid requirements for fluid resuscitation in our mouse model of TBI+HS vs. LR or Hextend. Also, in vitro experiments showed lack of the anticipated Hb neurotoxicity and surprising benefit in glutamate and neuronal stretch injury models, paralleling in vivo neuroprotection.29 Given the reduced fluid requirements in the mice treated with PNPH, we hypothesized that resuscitation with PNPH would reduce clinically relevant ICP and brain edema and improve CPP vs. LR in our mouse model of TBI+HS.
Materials and methods
The Institutional Animal Care and Use Committee of the University of Pittsburgh School of Medicine approved the experiments used in this study. The experiments were also conducted in a manner that addressed the key elements of the ARRIVE Guidelines. C57/BL6 mice (n=20) (Jackson Laboratories, Bar Harbor, ME, USA), 12 to 15 weeks of age, weighing 28.5±0.5 g were allowed ad libitum food and water and were housed in controlled environmental conditions until the study began. We randomized mice into two groups: standard resuscitation with LR or resuscitation with PNPH.
Anesthesia was induced with 4% isoflurane in 2:1N2O/oxygen delivered via nose cone and was then maintained with 1.5% isoflurane in a 2:1N2O/oxygen mixture for surgical preparation. Under sterile conditions an inguinal cutdown was performed and femoral venous and arterial catheters (modified PE-50 tubing) were placed. Mice were then placed in a stereotactic frame (Kopf, Tujunga, CA, USA) and an incision was made over the left scalp. Using a dental drill, a 5-mm craniotomy was performed over the left parietal cortex and the bone flap was removed. A brain temperature probe (Physitemp, Clifton, NJ, USA) was inserted through a burr hole in the left frontal cortex to monitor brain temperature, and body temperature was monitored via rectal probe. Brain temperature was controlled throughout the study via heating lamp to maintain temperature at 37±0.5°C. An ICP transducer (1 Tr MIKRO-TIP, Millar, Houston, TX, USA) was inserted (through a burr hole into the right frontal lobe, contralateral to injury). After craniotomy, the isoflurane was decreased to 1% in room air for 10 minutes before introduction of controlled cortical impact (CCI). A CCI shown previously to cause a mild-to-moderate injury level without significant mortality6 was performed with a pneumatic impactor (Bimba, Monee, IL, USA), using a flat 3-mm tip impounder on the left parietal cortex at 5 m/s and to a depth of 1.0 mm. Immediately after the injury, the craniotomy was replaced and sealed with dental cement and the scalp was closed.
Controlled Cortical Impact and Pressure Controlled Hemorrhagic Shock Model
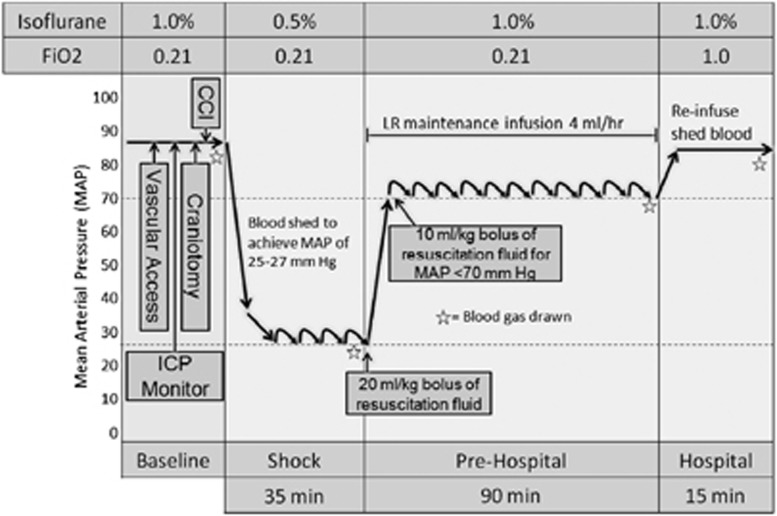
After CCI, mice underwent an HS paradigm (Figure 1) that has been used successfully in our laboratory for prior investigations.29, 30 Our model is designed to be clinically relevant, mimicking a TBI sustained in the field followed by HS. It consists of three phases, including ‘Shock,' ‘Prehospital,' and ‘Hospital.' Shock was initiated 5 minutes after CCI by removal of 12 mL of blood per kg of body weight over 5 minutes via the venous canula. Blood was drawn into a 1-mL tuberculin syringe containing 0.07 mL of citrate anticoagulant (Cardian BCT, Lakewood, CO, USA). An additional 12 mL of blood per kg of body weight was removed over the next 5 to 15 minutes for a total of ∼30% of the blood volume in a mouse.31 A mean arterial pressure (MAP) of 25 to 27 mm Hg was achieved and maintained by continued removal or reinfusion of autologous citrated blood from the venous catheter in 0.05 mL aliquots using the same syringe. The mice were maintained at this MAP for the duration of the 35-minute ‘Shock' phase initiated at the time of blood removal. Longer durations of the HS phase after CCI at this MAP level resulted in acute mortality in some mice in pilot studies; and thus, the 35-minute HS duration was selected as the longest duration reproducibly achievable with a low mortality rate. After the ‘Shock' phase, the mice entered the ‘Prehospital' phase, and were resuscitated with either LR or a 4% PNPH solution in normal saline (SynZyme Technologies, Irvine, CA, USA). Ten mice were assigned to each resuscitation group for a total of 20 mice. Resuscitation to a goal MAP of ⩾70 mm Hg was begun with an initial 20 mL/kg bolus of either PNPH or LR. Additional 10 mL/kg boluses of their respective fluids were administered every 5 minutes for an MAP of <70 mm Hg. For reference, the normal MAP in isoflurane anesthetized mice is ∼85 mm Hg in our preparation; a value of 70 mm Hg was selected based on the generally accepted goal of attempting to maintain cerebral perfusion pressure (CPP=MAP−ICP) of at least 60 mm Hg. Intracranial pressure in our model during the HS phase is low and generally ranges between 2 and 10 mm Hg (unpublished data). Mice in both groups were additionally administered a continuous maintenance infusion of LR at 4 mL/kg per hour for the duration of the model. The ‘Hospital' phase followed the 90-minute ‘Prehospital' phase wherein previously shed blood was reinfused over 15 minutes, simulating definitive care in a hospital setting. During the ‘Shock' phase, anesthesia was reduced from 1% isoflurane in room air to 0.5% isoflurane in room air. During the ‘Prehospital' phase, room air was used to mimic field resuscitation in the context of combat casualty care, where oxygen is not readily available. During the ‘Hospital' phase, 1% isoflurane in pure oxygen was administered, again mimicking clinical care in a combat support hospital or emergency department.
Figure 1.
Schematic of traumatic brain injury plus hemorrhagic shock (TBI+HS) model. This three-phase model, designed to be clinically relevant, involves an initial controlled cortical impact (CCI) immediately followed by a 35-minute ‘Shock' phase in which blood is withdrawn to achieve MAPs between 25 and 27 mm Hg. This is followed by a ‘Prehospital' resuscitation phase for 90 minutes, simulating filed resuscitation. In the ‘Prehospital' resuscitation phase, mice receive an initial 20 mL/kg bolus of fluid, followed by 10 mL/kg boluses every 5 minutes to maintain MAP ⩾70 mm Hg. Mice additionally receive a 4 mL/kg per hour maintenance infusion of lactated Ringer's (LR) during the ‘Prehospital' phase. Mice then enter the ‘Hospital' phase where they receive reinfusion of shed blood, simulating transfusion in the field hospital or emergency department. ICP, intracranial pressure.
At the completion of the protocol, the mice were decapitated and their brains were immediately removed and bisected into left and right hemispheres. Each hemisphere was immediately weighed (wet weight). Hemispheres were then placed in an oven, dehydrated at 110°C for 48 hours, and reweighed (dry weight). Percent brain water (%BW) content of injured and contralateral hemispheres was determined by the wet–dry weight method.32 To establish baseline %BW in naive animals, an additional nine uninjured mice were anesthetized and decapitated and %BW was determined.
Monitoring During Controlled Cortical Impact+Hemorrhagic Shock Model
Mean arterial pressure was continuously monitored and recorded via the femoral arterial catheter at baseline, after CCI, and every 5 minutes during HS and resuscitation. Heart rate was similarly monitored and recorded. Additionally, we continuously monitored ICP in these mice using a 1 Fr MIKRO-TIP transducer (Millar, Houston, TX, USA) as previously reported in our model.30 Laboratory evaluation with arterial blood gases, lactate, glucose, hematocrit, Hb, sodium, potassium, calcium, magnesium, blood urea nitrogen, and osmolarity (Model ABL-90 Radiometer America, Westlake, Ohio) was obtained at four time points: baseline, 30 minutes into the ‘Shock' phase, at the end of the ‘Prehospital' phase, and at the end of the ‘Hospital' phase.
Statistics
Physiologic parameters were analyzed by two-way ANOVA for repeated measures and between group comparisons for other outcomes were made using Student's T-test. Post hoc testing was corrected for multiple comparisons. All results are provided as mean±s.e.m. Correlations between %BW in the injured hemispheres and key physiologic parameters during resuscitation (mean ICP, peak ICP, ‘Hospital Phase' ICP, and resuscitation fluid volumes) were assessed using Spearman rank correlation. Significance was determined by a P value of ⩽0.05.
Results
Mortality
Once resuscitation was begun, all mice survived until the end of the model, at which time they were killed. One mouse died during the ‘Shock' phase before randomization and was therefore excluded from analysis.
Hemodynamics
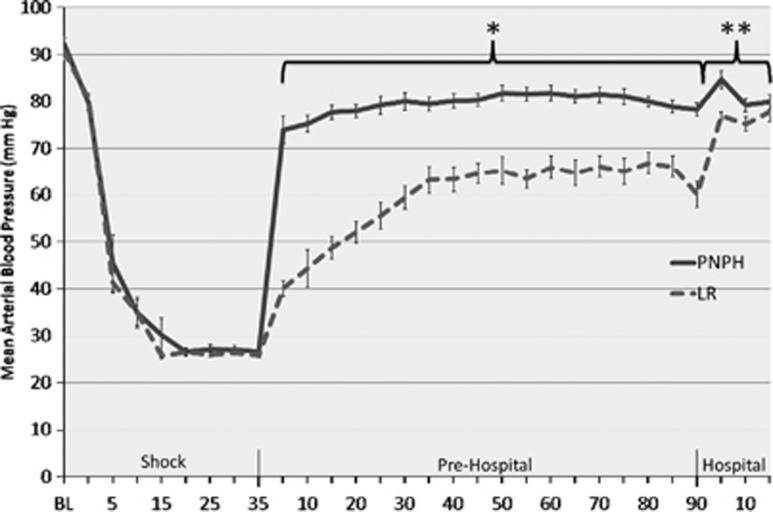
There was no significant difference in MAP at baseline or during the ‘Shock' phase between treatment groups (Figure 2). Both groups achieved the target MAP (25 to 27 mm Hg) within 20 minutes of the initiation of HS and maintained this MAP for the duration of the phase. There was no significant difference in the blood volume removed to achieve target MAP (27.1±0.98 vs. 27.1±0.92 mL/kg). Mean MAP during ‘Prehospital' resuscitation for the PNPH-resuscitated mice was higher than LR (79.4±0.40 vs. 59.7±0.83 mm Hg, P<0.001). After initiation of resuscitation in the ‘Prehospital' phase, the PNPH-resuscitated mice had an immediate restoration of their MAP to the goal of ⩾70 mm Hg. Mean arterial pressure did not decrease <70 mm Hg in mice resuscitated with PNPH after the initial bolus and throughout the remainder of the model. In contrast, at no time point did the mice resuscitated with LR achieve a mean MAP of >70 mm Hg until reinfusion of shed blood. After reinfusion of the shed blood in the ‘Hospital' phase, MAP improved to >70 mm Hg in both treatment groups, however, the PNPH-resuscitated mice continued to have higher mean MAPs vs. LR (81.3±1.05 vs. 76.6±0.88, P<0.01).
Figure 2.
Mean arterial blood pressure of mice during model. Mice resuscitated with polynitroxylated-pegylated hemoglobin (PNPH) were normotensive and had significantly higher blood pressure vs. lactated Ringer's (LR)-resuscitated mice at all time points immediately on initiation of resuscitation and the duration of the ‘Prehospital' phase (*P<0.001). After reinfusion of shed blood in the ‘Hospital' phase, PNPH-resuscitated mice continued to have significantly higher blood pressures vs. LR-resuscitated mice (**P<0.001). BL, baseline.
Fluid Requirements for Resuscitation
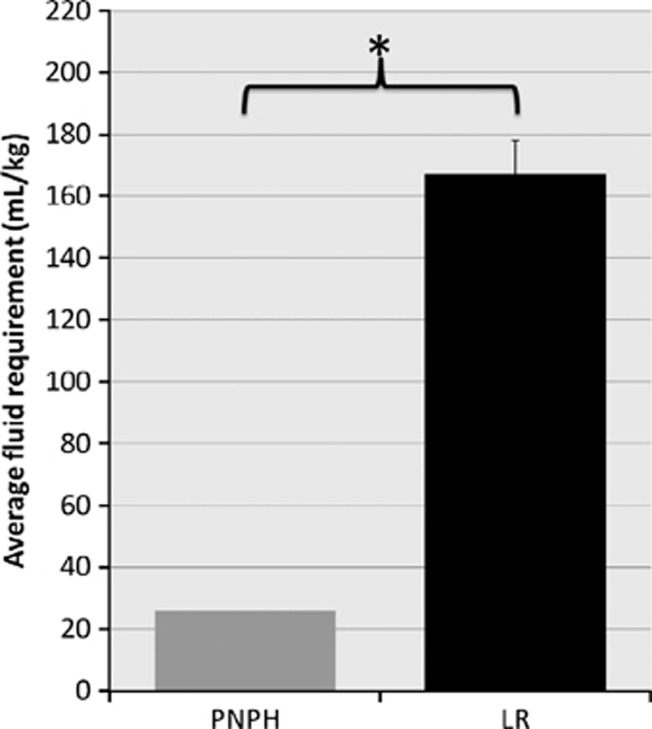
Mice resuscitated with PNPH had a significantly reduced fluid requirement during the ‘Prehospital' resuscitation phase (Figure 3). Mice resuscitated with PNPH required 26.0±0 mL/kg for total fluid resuscitation, whereas LR-resuscitated mice required 167.0±10.69 mL/kg (P<0.001). Mice resuscitated with PNPH required only the initial bolus of 20 mL/kg at the beginning of the resuscitation phase, and were then maintained on the LR maintenance infusion, accounting for the additional 6 mL/kg received. The PNPH-resuscitated mice all had a MAP of >70 mm Hg after the initial bolus of resuscitation fluid and did not require additional boluses as per the study protocol. In contrast, the LR-resuscitated mice required an average of 14 out of a total of 18 possible fluid boluses during the ‘Prehospital' phase.
Figure 3.
Mean fluid requirements for resuscitation. Fluid requirements in ‘Prehospital' phase were significantly less in polynitroxylated-pegylated hemoglobin (PNPH)-resuscitated mice compared with lactated Ringer's (LR) (26±0 vs. 167±10.7 mL/kg, *P<0.001). The PNPH-resuscitated mice required only one bolus of PNPH while LR-resuscitated mice on average required fluid boluses 14 of 18 possible times. Both groups were run on a continuous maintenance infusion of LR at 4 mL/kg per hour during the 90-minute ‘Prehospital' phase accounting for an additional 6 mL/kg per group.
Intracranial Pressure and Cerebral Perfusion Pressure
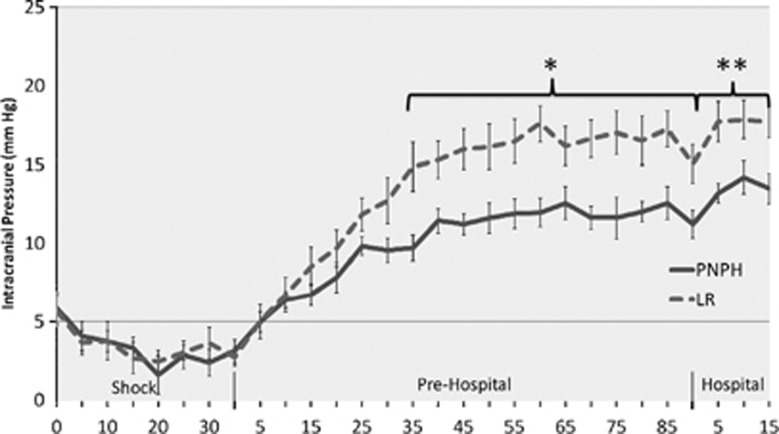
Mice at baseline and during the initial portions of the model had no significant differences in ICP (Figure 4). Mice resuscitated with LR began to have increased ICP vs. those resuscitated with PNPH at 35 minutes into the 90-minute ‘Prehospital' resuscitation phase (14.9±1.6 vs. 9.7±0.82 mm Hg, P=0.01), which persisted at each recorded time point for the remainder of the model. Mean ICP for mice resuscitated with LR was higher vs. PNPH in both the ‘Prehospital' phase (13.9±0.40 versus 10.3±0.25 mm Hg, P=0.004) and the ‘Hospital' phase (17.8±0.65 vs. 13.6±0.51 mm Hg, P<0.001). Mean peak ICP was also higher in the LR-resuscitated mice vs. PNPH (19.7±1.12 vs. 14.5±0.97 mm Hg, P=0.002).
Figure 4.
Intracranial pressure (ICP) of mice during model. Mice resuscitated with lactated Ringer's (LR) compared with polynitroxylated-pegylated hemoglobin (PNPH) had significantly elevated ICP beginning 35 minutes into the ‘Prehospital' phase and continued to have significantly elevated ICP the duration of the model (P<0.01). For LR vs. PNPH, average ‘Prehospital' ICP was 13.98±0.40 vs. 10.27±0.25 mm Hg (*P=0.004), and average ‘Hospital' phase ICP was 17.7±0.99 vs. 13.5±0.96 mm Hg (**P=0.002), respectively.
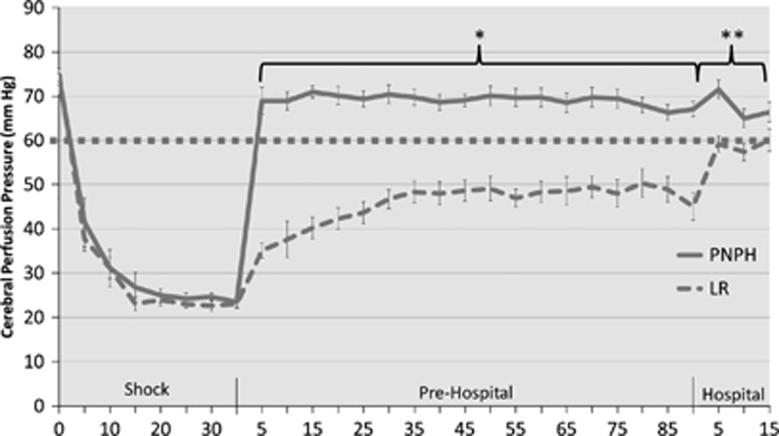
Cerebral perfusion pressure at baseline and during HS was not significantly different between groups. During the ‘Shock' phase, CPP in both groups quickly decreased to ∼23 to 24 mm Hg and remained at that level for the duration of the phase (Figure 5). On initiation of resuscitation in the ‘Prehospital' phase, CPP in the PNPH-resuscitated mice was rapidly restored to >60 mm Hg (typically regarded as target threshold in adult TBI patients) and remained above this threshold for the duration of the model. In contrast, mice resuscitated with LR remained well below this threshold for the duration of the ‘Prehospital' phase. In addition, mice resuscitated with LR did not achieve a CPP of ⩾60 mm Hg until the final time point in the ‘Hospital' phase. Mean CPP in the ‘Prehospital' phase for PNPH-resuscitated mice was 69.2±0.46 vs. 45.5±0.68 mm Hg for the LR group (P<0.001). Mean CPP in the ‘Hospital' phase for the PNPH-resuscitated mice was 67.7±1.30 vs. 58.5±1.22 mm Hg for LR (P<0.001).
Figure 5.
Cerebral perfusion pressure (CPP) (MAP-ICP) of mice during model. Polynitroxylated-pegylated hemoglobin (PNPH)-resuscitated mice had immediate restoration of CPP above clinically relevant goal of >60 mm Hg (dotted line), while mice resuscitated with LR never achieved this threshold until reinfusion of shed blood. Average ‘Prehospital' CPP for PNPH vs. LR was 69.2±0.46 vs. 45.5±0.68 mm Hg, *P<0.001. After reinfusion of shed blood in the ‘Hospital' phase, PNPH-resuscitated mice continued to have significantly higher CPP vs. LR (67.7±1.3 vs. 58.5±1.22, **P<0.001). ICP, intracranial pressure; MAP, mean arterial pressure.
Brain Edema
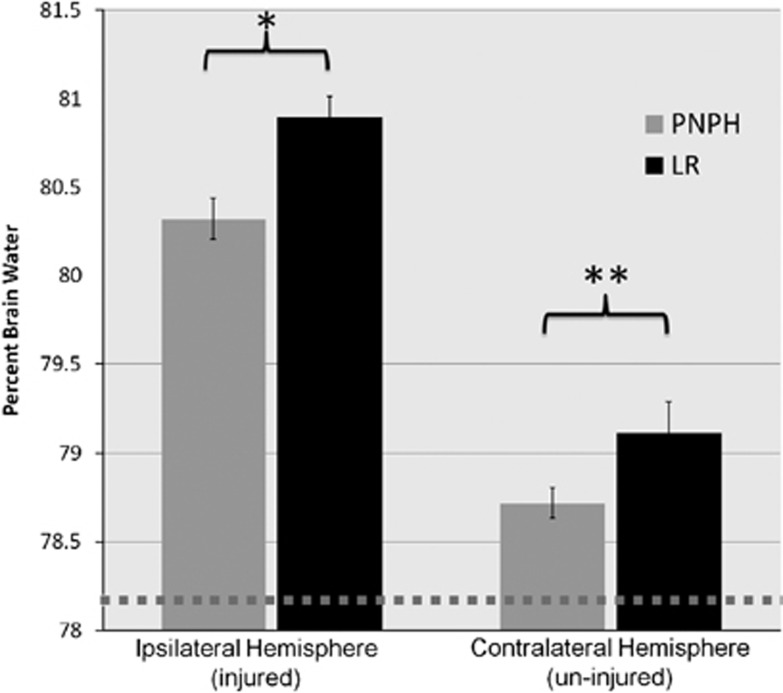
Percent brain water as a marker of brain edema (Figure 6) was significantly reduced in the ipsilateral (injured) hemisphere of the mice resuscitated with PNPH vs. those resuscitated with LR (80.3±0.12% vs. 80.9±0.12%, P=0.003). The contralateral hemisphere of mice resuscitated with PNPH also had a numerically lower %BW vs. LR, though it did not reach statistical significance (78.7±0.08% vs. 79.1±0.17%, P=0.07). Additionally, both groups had a significantly higher %BW than those of uninjured mice (78.2±0.06%, P<0.001).
Figure 6.
Percent brain water (%BW) by wet/dry weight method. Percent brain water was significantly lower in the ipsilateral (injured) hemisphere in polynitroxylated-pegylated hemoglobin (PNPH)-resuscitated mice vs. lactated Ringer's (LR) (80.3±0.12% vs. 80.9±0.12%, *P=0.003) and was numerically lower, though not statistically significant in the contralateral (uninjured) hemisphere for PNPH vs. LR (78.7±0.08% vs. 79.1±0.17%, **P=0.07). Both groups did have increased %BW from that of uninjured mice (78.2±0.06%, shown by dotted line).
Relationship Between Brain Edema and Resuscitation Physiology
Of the key physiologic parameters assessed during resuscitation, both peak ICP and resuscitation fluid volumes were significantly correlated with brain edema as assessed by %BW in the injured hemisphere (R=0.568, P=0.014 and R=0.718, P=0.000, respectively).
Blood Gas and Chemistry Analysis
Blood gas analysis (Table 1) revealed small differences between groups. As expected, both groups experienced a significant lactic acidosis immediately after the ‘Shock' phase, however, by the end of the model both groups had largely cleared this lactate. Peak lactate (Table 1) for the PNPH-resuscitated mice was numerically lower vs. LR (8.56±0.81 vs. 10.07±1.19 mmol/L), although this was not statistically significant (P=0.30). Overall, there was no significant difference in either group's acid–base status. Oxygen saturations in the LR-resuscitated mice were significantly lower at the end of the ‘Prehospital' phase vs. PNPH (94.85±0.94% vs. 98.82±0.58%, P=0.002). Additionally, PaO2 was numerically lower in the LR-resuscitated mice as well, although it was not significantly different (82.54±2.40 vs. 87.63±2.62 mm Hg, P=0.17).
Table 1. Physiologic parameters.
| Baseline | End HS | End Pre-hospital | End hospital | |
|---|---|---|---|---|
| Brain temperature (°C) | ||||
| PNPH | 37.37±0.10 | 37.39±0.13 | 37.36±0.12 | 37.19±0.06 |
| LR | 37.37±0.05 | 37.39±0.05 | 37.36±0.05 | 37.19±0.05 |
| pH | ||||
| PHPN | 7.35±0.01 | 7.30±0.03 | 7.41±0.01 | 7.30±0.01 |
| LR | 7.34±0.01 | 7.26±0.04 | 7.37±0.01 | 7.27±0.02 |
| pCO2 (mm Hg) | ||||
| PHPN | 40.87±1.76 | 24.63±0.74 | 34.18±1.51 | 48.01±1.26 |
| LR | 43.49±1.33 | 26.36±2.47 | 37.92±1.24 | 49.83±3.22 |
| pO2 (mm Hg) | ||||
| PHPN | 141.60±5.7 | 99.59±1.87 | 87.63±2.62 | 506.90±5.98 |
| LR | 149.50±2.67 | 100.79±1.43 | 82.54±2.40 | 494.11±16.43 |
| Oxygen saturation (%) | ||||
| PHPN | 97.81±0.43 | 94.43±0.80 | 98.82±0.58 | 100.0±0 |
| LR | 98.14±0.18 | 90.92±2.34 | 94.85±0.94 | 99.74±0.26 |
| Hematocrit (%) | ||||
| PHPN | 43.83±0.48 | 26.99±2.32 | 20.39±0.64 | 28.20±0.59 |
| LR | 43.60±0.28 | 29.29±0.57 | 19.75±0.62 | 28.98±0.60 |
| Hemoglobin (g/dL) | ||||
| PHPN | 14.29±0.16 | 9.46±0.30 | 6.64±0.20 | 9.20±0.19 |
| LR | 14.15±0.08 | 9.59±0.18 | 6.42±0.20 | 9.45±0.20 |
| Base excess (mEq/L) | ||||
| PHPN | −2.65±0.40 | −12.91±0.84 | −1.74±0.46 | −2.43±0.37 |
| LR | −2.34±0.21 | −14.64±1.37 | −2.98±0.61 | −4.26±0.83 |
| Bicarbonate (mmol/L) | ||||
| PHPN | 20.15±2.04 | 12.29±0.53 | 22.82±0.47 | 23.72±0.37 |
| LR | 23.82±0.41 | 11.17±0.89 | 22.01±0.61 | 22.35±0.71 |
| Sodium (mmol/L) | ||||
| PHPN | 146.70±0.62 | 142.30±0.72 | 145.00±0.45 | 145.40±0.43 |
| LR | 147.60±0.45 | 146.60±0.39 | 141.50±0.27 | 141.90±0.38 |
| Potassium (mmol/L) | ||||
| PHPN | 4.64±0.08 | 6.25±0.18 | 4.79±0.11 | 4.68±0.07 |
| LR | 4.72±0.06 | 6.36±0.20 | 5.10±0.21 | 5.19±0.22 |
| Chloride (mmol/L) | ||||
| PHPN | 116.04±0.56 | 118.80±0.99 | 123.90±2.96 | 120.20±0.47 |
| LR | 116.60±0.50 | 119.10±0.60 | 117.10±0.46 | 117.10±0.38 |
| Calcium (mmol/L) | ||||
| PHPN | 1.14±0.03 | 1.11±0.02 | 1.13±0.03 | 1.08±0.03 |
| LR | 1.17±0.02 | 1.13±0.03 | 1.12±0.02 | 1.03±0.05 |
| Glucose (mg/dL) | ||||
| PHPN | 262.50±11.15 | 420.90±33.31 | 166.00±30.02 | 126.00±3.31 |
| LR | 228.50±11.42 | 359.50±23.37 | 143.60±16.43 | 129.10±11.91 |
| Lactate (mmol/L) | ||||
| PHPN | 2.31±0.24 | 8.56±0.81 | 1.70±0.08 | 1.19±0.06 |
| LR | 2.18±0.13 | 10.07±1.19 | 2.23±0.31 | 1.43±0.25 |
| Osmolality (mmol/kg) | ||||
| PHPN | 307.30±0.63 | 306.42±1.69 | 297.61±1 | 297.77±0.98 |
| LR | 307.72±0.69 | 308.00±1.79 | 290.86±0.84 | 290.29±1.28 |
| Methemoglobinemia (%) | ||||
| PHPN | 0±0 | 0±0 | 4.64±0.27 | 1.44±0.18 |
| LR | 0±0 | 0±0 | 0.05±0.02 | 0±0 |
Abbreviations: HS, hemorrhagic shock; LR, lactated Ringer's; PNPH, polynitroxylated-pegylated hemoglobin.
All values are mean±standard error of the mean.
Both groups showed hyperglycemia (360 to 421 mg/dL) at the end of the ‘Shock' phase (Table 1), though there was no significant difference between groups (P=0.15). Also, at the end of the ‘Shock' phase, serum potassium levels were increased in both groups (6.25 to 6.36 mmol/L), likely from extracellular shifts secondary to acidosis. Not surprisingly, given the large volume of resuscitation in the LR group, there were slight, but statistically significant reductions in serum sodium and chloride at the end of both the ‘Prehospital' and ‘Hospital' phases in the LR-resuscitated mice vs. PNPH (Table 1), though this difference is not likely clinically significant. Osmolarity decreased over the course of the model in both groups. However, at the end of the ‘Hospital' phase the LR-resuscitated mice had a lower osmolarity than those resuscitated with PNPH (290.29±1.28 vs. 297.77±0.98 mmol/L, P<0.001).
Hemoglobin and hematocrit (Table 1) were significantly decreased after the ‘Shock' phase and continued to decrease over the course of the ‘Prehospital' resuscitation. Both groups' baseline Hb levels approximated 14 g/dL; at the end of the ‘Prehospital' phase they had decreased to ∼6 g/dL; and after reinfusion of shed blood Hb levels increased to around 9 g/dL. Remarkably, there were no significant differences between the groups' Hb or hematocrit at any of the time points. This is in spite of the LR-resuscitated mice receiving over 140 mL/kg more fluid for resuscitation than the PNPH mice. Of note, PNPH is a 4% Hb-based solution (corresponding to an Hb concentration of 4 g/dL).
Given the possible concern about the production of MetHb with nitroxides and HBOCs, MetHb levels were followed during blood gas analysis (Table 1). Both groups had no detectable MetHb at baseline or at the end of the ‘Shock' phase. The PNPH-treated mice developed increased MetHb levels at the end of the ‘Prehospital' phase (4.64±0.27% vs. 0.05±0.02%, P>0.001). After entering the ‘Hospital' phase MetHb levels decreased, however remained above those of the LR-treated mice (1.44±0.18% vs.0±0%, P<0.001). No individual mouse had an MetHb level of >6%.
Discussion
Resuscitation with PNPH in our mouse model of TBI+HS showed advantages over traditional resuscitation with LR. Mice resuscitated with PNPH had reduced ICP, improved CPP, and attenuated brain edema vs. mice resuscitated with LR. We also confirmed our prior findings that resuscitation with PNPH immediately restores MAP and CPP to target thresholds and is associated with a significant reduction in required fluid volume.29 Unlike our prior study, however, LR-resuscitated mice never achieved the more rigorous MAP or CPP goals during the resuscitation period in spite of receiving markedly more volume for resuscitation. This likely relates to the much more severe HS used in our current experiment. In a similar model of TBI+HS, we previously reported that sustained hypotension after TBI exacerbates hippocampal neuronal loss,6 thus underscoring the advantage of prompt resuscitation. Overall, these effects, if seen in a human clinical trial, could be clinically relevant.
Polynitroxylated-pegylated hemoglobin appears to have excellent resuscitative properties in TBI+HS with immediate restoration of MAP. This could be attributed to the vasoconstrictive properties of many prior generation HBOCs from either NO consumption or excessive prearteriolar oxygen delivery,21 however, our data suggest otherwise. Our data argue against significant vasoconstriction from NO scavenging by PNPH given the recovery of normal acid–base status and lactate levels at the end of the ‘Prehospital' and ‘Hospital' phases and our prior report showing neuroprotection.29 This suggests adequate tissue perfusion, though the possibility of a mild vasopressor effect cannot be excluded. The nitroxylation of Hb in PNPH serves to directly limit the NO scavenging effect of free Hb.26, 27, 33 Also, the pegylation of Hb, while likely conferring significant colloidal effects, could also function as a physical barrier to the interaction of the Hb with the vascular endothelium, and its large molecular size (110 kDa) could increase the likelihood of PNPH remaining within the vasculature34—reducing its interaction with NO.
With regard to the proposed efficacy of PNPH as a small volume resuscitation fluid in TBI+HS, our findings suggest substantial benefit on numerous clinically relevant parameters that may result directly from the aforementioned modifications of PNPH. At the end of the ‘Shock' and ‘Prehospital' phases, both LR and PNPH groups surprisingly had no difference in Hb values, in spite of the fact that the LR group received a sixfold greater resuscitation volume than the PNPH group. Such a large volume of resuscitation undoubtedly contributed to the dilutional anemia that was seen after resuscitation from severe HS. However, the Hb values were not higher in the PNPH mice. This supports a purported ‘super-colloid' effect of PNPH. Each molecule of polyethylene glycol has been suggested to bind theoretically as many as 132 molecules of water; thus, each PNPH molecule could potentially bind >1,000 water molecules, expanding the vascular space and reducing interstitial water including potentially brain water.29 Our data suggest a marked oncotic effect of PNPH, given the fact that it compensated, at least acutely, for a sixfold difference in resuscitation volume vs. LR. This could be a promising effect in TBI resuscitation.
The reduced fluid requirements for the PNPH-treated mice appeared to directly benefit the traumatically injured brain. Large resuscitation volumes can exacerbate cerebral edema and fail to restore oxygen delivery.12 Furthermore, volume-limiting resuscitation strategies with hypertonic or colloidal-based solutions, or even a combination of fluid and vasopressors35 have been shown to have potential central nervous system protective effects, with reduced ICP and brain edema as well as improved CPP. These findings parallel our observations, as we noted significantly reduced ICP and %BW with PNPH vs. LR resuscitation, along with improved CPP.13, 14 These findings are likely a result of either a reduction in resuscitation fluid volume, direct removal of water from the brain interstitial space, or reduced early ischemia which is seen in our model even with less severe MAP reductions than in this study.36 Indeed %BW was highly correlated with resuscitation fluid volume in our model. Also, mice resuscitated with PNPH may have some early benefits from reduced volume resuscitation outside the central nervous system. In our study, mice resuscitated with LR had significantly lower oxygen saturations and a trend toward reduced PaO2, which may reflect some evidence of early pulmonary edema. There is increasing evidence that resuscitation strategies based primarily on aggressive crystalloid use are associated with cardiac and pulmonary complications (including pulmonary edema), gastrointestinal dysmotility, and coagulation disturbances.37 Although the difference in arterial saturation between the groups of ∼98 vs. ∼94% may not seem to be of great clinical significance, given the potentially increased diffusion distance in the edematous brain after TBI, an effect of this level of difference in SaO2 cannot be dismissed. Brain tissue oxygen monitoring could help define the impact of this finding.
The PNPH-resuscitated mice had increased whole blood MetHb levels vs. LR by the end of the ‘Prehospital' resuscitation phase. After reinfusion of the shed blood in the ‘Hospital' phase, these levels had greatly decreased, however, they remained significantly elevated above baseline. This most likely reflects the direct measurement of the PNPH itself in solution. Before infusion, PNPH has a baseline MetHb level of 6% to 7%. A final MetHb concentration of 3% to 4% would is expected in our model by infusing a 4% PNPH solution (with a 7% MetHb concentration) to a mouse with an initial Hb level of 9.5 g/dL. This is in keeping with our findings. Measurements in this experiment were taken from whole blood samples, which therefore would include extracellular Hb. Separate measurement of serum and cellular MetHb concentrations would provide conclusive evidence regarding the etiology of the elevated MetHb concentrations; however, this was not undertaken during this current study.
Limitations of the Study
Our model was designed to represent a paradigm relevant to both civilian and military personnel in the treatment of TBI plus severe HS. Current practices in the resuscitation of polytrauma and hemorrhage vary, however, some institutions practice ‘controlled hypotension,' targeting MAPs of 50 to 60 mm Hg: lower than the target of 70 mm Hg in our model. The concept of controlled hypotension was based on the findings of Bickell et al38 who found in a prospective trial of penetrating abdominal injuries that delaying aggressive fluid resuscitation until operative intervention improved survival. However, TBI is considered as a contraindication to the controlled hypotension approach; thus, the MAP target of 70 mm Hg was chosen to represent the low end of normal for mice.38, 39 Our approach is also in line with the Guidelines for the Management of Severe TBI.40 Nevertheless, the optimum MAP after TBI has not been established and the selection of a lower target MAP may well have led to different results in our study, particularly volume requirements for LR resuscitation. Indeed, the optimal BP could vary depending on a number of factors such as severity or type of TBI or the duration of hypotension. Also related to our desire to produce a clinically relevant scenario, we did not resuscitate with shed blood in the ‘Prehospital' phase, given the lack of availability of blood products in the field. We also did not measure the impact of PNPH on the CBF after resuscitation in this model.
Our study only addressed the acute phase of TBI management, terminating on completion of resuscitation. Studies providing longer term follow-up would provide additional, valuable information, however, the current scope of this project was to evaluate the acute characteristics of PNPH resuscitation. We did not measure neuropathology at the end of the resuscitation in this study, however, we have previously published, in a similar model of TBI+HS, reduced neuronal death in the hippocampus at 7 days after resuscitation with PNPH vs. either LR or hetastarch, corresponding to improved cell viability in this vulnerable area.29 We have also reported neuroprotection rather than neurotoxicity by PNPH in vitro in neuronal culture models. Nevertheless, our current study suggests added benefit on clinically relevant aspects of acute cerebrovascular physiology. Further research is warranted focusing on long-term behavioral and histopathologic outcomes.
In conclusion, resuscitation with PNPH after TBI, to a clinically relevant MAP target, requires dramatically less fluid vs. LR, reduces ICP, better maintains CPP, and reduces brain edema. Polynitroxylated-pegylated hemoglobin also greatly enhanced the speed of restoration of MAP and CPP vs. LR, which could represent another important therapeutic finding. Our data build on prior findings and provide evidence of benefit of PNPH on clinically relevant parameters in the acute resuscitation phase. Our data support ongoing preclinical development of this novel resuscitation fluid for TBI resuscitation.
Acknowledgments
The authors thank NINDS U44 NS070324 (CJCH and PMK) and NICHD (T32 HD040686 [SLS], K12 HD047349 [ELF]) for support. The authors thank Marci Provins for manuscript preparation.
Both Drs Hsia and Ma are shareholders in SynZyme Technologies, LLC.
Footnotes
This study was supported by U44 NS070324, T32 HD040686, and K12 HD047349.
References
- Chesnut RM. Secondary brain insults after head injury: clinical perspectives. New Horiz. 1995;3:366–375. [PubMed] [Google Scholar]
- Nelson TJ, Wall DB, Stedje-Larsen ET, Clark RT, Chambers LW, Bohman HR. Predictors of mortality in close proximity blast injuries during Operation Iraqi Freedom. J Am Coll Surg. 2006;202:418–422. doi: 10.1016/j.jamcollsurg.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Jenkins LW, Moszynski K, Lyeth BG, Lewelt W, DeWitt DS, Allen A, et al. Increased vulnerability of the mildly traumatized rat brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Res. 1989;477:211–224. doi: 10.1016/0006-8993(89)91409-1. [DOI] [PubMed] [Google Scholar]
- Ishige N, Pitts LH, Berry I, Nishimura MC, James TL. The effects of hypovolemic hypotension on high-energy phosphate metabolism of traumatized brain in rats. J Neurosurg. 1988;68:129–136. doi: 10.3171/jns.1988.68.1.0129. [DOI] [PubMed] [Google Scholar]
- Hellmich HL, Garcia JM, Shimamura M, Shah SA, Avila MA, Uchida T, et al. Traumatic brain injury and hemorrhagic hypotension suppress neuroprotective gene expression in injured hippocampal neurons. Anesthesiology. 2005;102:806–814. doi: 10.1097/00000542-200504000-00017. [DOI] [PubMed] [Google Scholar]
- Dennis AM, Haselkorn ML, Vagni VA, Garman RH, Janesko-Feldman K, Bayir H, et al. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J Neurotrauma. 2009;26:889–899. doi: 10.1089/neu.2008.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroppenstedt SN, Kern M, Thomale UW, Schneider GH, Lanksch WR, Unterberg AW. Effect of cerebral perfusion pressure on contusion volume following impact injury. J Neurosurg. 1999;90:520–526. doi: 10.3171/jns.1999.90.3.0520. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Bramlett HM, Kuluz JW, Alonso O, Dietrich WD. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathologic consequences of traumatic brain injury in rats. J Cereb Blood Flow Metab. 2001;21:847–856. doi: 10.1097/00004647-200107000-00010. [DOI] [PubMed] [Google Scholar]
- DeWitt DS, Prough DS, Taylor CL, Whitley JM. Reduced cerebral blood flow, oxygen delivery, and electroencephalographic activity after traumatic brain injury and mild hemorrhage in cats. J Neurosurg. 1992;76:812–821. doi: 10.3171/jns.1992.76.5.0812. [DOI] [PubMed] [Google Scholar]
- DeWitt DS, Prough DS, Taylor CL, Whitley JM, Deal DD, Vines SM. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am J Physiol. 1992;263 (4 Pt 2:H1276–H1284. doi: 10.1152/ajpheart.1992.263.4.H1276. [DOI] [PubMed] [Google Scholar]
- Graham DI, Adams JH. Ischaemic brain damage in fatal head injuries. Lancet. 1971;1:265–266. doi: 10.1016/s0140-6736(71)91003-8. [DOI] [PubMed] [Google Scholar]
- Ramming S, Shackford SR, Zhuang J, Schmoker JD. The relationship of fluid balance and sodium administration to cerebral edema formation and intracranial pressure in a porcine model of brain injury. J Trauma. 1994;37:705–713. doi: 10.1097/00005373-199411000-00003. [DOI] [PubMed] [Google Scholar]
- Jungner M, Grande PO, Mattiasson G, Bentzer P. Effects on brain edema of crystalloid and albumin fluid resuscitation after brain trauma and hemorrhage in the rat. Anesthesiology. 2010;112:1194–1203. doi: 10.1097/ALN.0b013e3181d94d6e. [DOI] [PubMed] [Google Scholar]
- Schmoker JD, Zhuang J, Shackford SR. Hypertonic fluid resuscitation improves cerebral oxygen delivery and reduces intracranial pressure after hemorrhagic shock. J Trauma. 1991;31:1607–1613. doi: 10.1097/00005373-199112000-00007. [DOI] [PubMed] [Google Scholar]
- Bulger EM, May S, Brasel KJ, Schreiber M, Kerby JD, Tisherman SA, et al. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA. 2010;304:1455–1464. doi: 10.1001/jama.2010.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- Reid TJ. Hb-based oxygen carriers: are we there yet. Transfusion. 2003;43:280–287. doi: 10.1046/j.1537-2995.2003.00314.x. [DOI] [PubMed] [Google Scholar]
- DeAngeles DA, Scott AM, McGrath AM, Korent VA, Rodenkirch LA, Conhaim RL, et al. Resuscitation from hemorrhagic shock with diaspirin cross-linked hemoglobin, blood, or hetastarch J Trauma 199742406–412.discussion 412-414. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr., et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta TC, Walker BR, Eichinger MR, Doyle MP. Rate of NO scavenging alters effects of recombinant hemoglobin solutions on pulmonary vasoreactivity. J Appl Physiol. 2002;93:1327–1336. doi: 10.1152/japplphysiol.00175.2002. [DOI] [PubMed] [Google Scholar]
- Burhop K, Gordon D, Estep T. Review of hemoglobin-induced myocardial lesions. Artif Cells Blood Substit Immobil Biotechnol. 2004;32:353–374. doi: 10.1081/bio-200027429. [DOI] [PubMed] [Google Scholar]
- Hsia CJ, Ma L. A hemoglobin-based multifunctional therapeutic: polynitroxylated pegylated hemoglobin. Artif Organs. 2012;36:215–220. doi: 10.1111/j.1525-1594.2011.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alayash AI. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants. Nat Biotechnol. 1999;17:545–549. doi: 10.1038/9849. [DOI] [PubMed] [Google Scholar]
- Buehler PW, Alayash AI. Toxicities of hemoglobin solutions: in search of in-vitro and in-vivo model systems. Transfusion. 2004;44:1516–1530. doi: 10.1111/j.1537-2995.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Saetzler RK, Arfors KE, Tuma RF, Vasthare U, Ma L, Hsia CJ, et al. Polynitroxylated hemoglobin-based oxygen carrier: inhibition of free radical-induced microcirculatory dysfunction. Free Radic Biol Med. 1999;27:1–6. doi: 10.1016/s0891-5849(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Stoyanovsky DA, Kapralov A, Huang Z, Maeda A, Osipov A, Hsia CJ, et al. Unusual peroxidase activity of polynitroxylated pegylated hemoglobin: elimination of H(2)O(2) coupled with intramolecular oxidation of nitroxides. Biochem Biophys Res Commun. 2010;399:139–143. doi: 10.1016/j.bbrc.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Shellington DK, Du L, Wu X, Exo J, Vagni V, Ma L, et al. Polynitroxylated pegylated hemoglobin: a novel neuroprotective hemoglobin for acute volume-limited fluid resuscitation after combined traumatic brain injury and hemorrhagic hypotension in mice. Crit Care Med. 2011;39:494–505. doi: 10.1097/CCM.0b013e318206b1fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemerka JN, Wu X, Dixon CE, Garman RH, Exo JL, Shellington DK, et al. Severe brief pressure-controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice. J Neurotrauma. 2012;29:2192–2208. doi: 10.1089/neu.2011.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches AC, Sharp JG, Thomas DB, Smith SV. Blood volume determination in the mouse. J Physiol. 1973;228:279–284. doi: 10.1113/jphysiol.1973.sp010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield RT, Schiding JK, Hamilton RL, Kochanek PM. Effects of hypothermia on traumatic brain injury in immature rats. J Cereb Blood Flow Metab. 1996;16:244–252. doi: 10.1097/00004647-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Okayama N, Park JH, Coe L, Granger DN, Ma L, Hisa CJ, et al. Polynitroxyl alphaalpha-hemoglobin (PNH) inhibits peroxide and superoxide-mediated neutrophil adherence to human endothelial cells. Free Radic Res. 1999;31:53–58. doi: 10.1080/10715769900300591. [DOI] [PubMed] [Google Scholar]
- Wills BA, Oragui EE, Dung NM, Loan HT, Chau NV, Farrar JJ, et al. Size and charge characteristics of the protein leak in dengue shock syndrome. J Infect Dis. 2004;190:810–818. doi: 10.1086/422754. [DOI] [PubMed] [Google Scholar]
- Feinstein AJ, Patel MB, Sanui M, Cohn SM, Majetschak M, Proctor KG. Resuscitation with pressors after traumatic brain injury. J Am Coll Surg. 2005;201:536–545. doi: 10.1016/j.jamcollsurg.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Foley LM, Iqbal O'Meara AM, Wisniewski SR, Kevin Hitchens T, Melick JA, Ho C, et al. MRI assessment of cerebral blood flow after experimental traumatic brain injury combined with hemorrhagic shock in mice. J Cereb Blood Flow Metab. 2013;33:129–136. doi: 10.1038/jcbfm.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton BA, Guy JS, Morris JA, Jr., Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- Bickell WH, Wall MJ, Jr., Pepe PE, Martin RR, Ginger VF, Allen MK, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- Morrison CA, Carrick MM, Norman MA, Scott BG, Welsh FJ, Tsai P, et al. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011;70:652–663. doi: 10.1097/TA.0b013e31820e77ea. [DOI] [PubMed] [Google Scholar]
- Bratton SL, Chesnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24 (Suppl 1:S1–106. [Google Scholar]