Abstract
Delayed cerebral vasospasm is thought to be caused by factors released from a subarachnoid blood clot. Because vasospasm occurs several days after hemorrhage, we hypothesized that clotted blood releases vasoactive factors as it ages. Targeted proteomics identified histidine-rich glycoprotein (HRG) as a potentially vasoactive factor released within the first 72 hours of clot formation. In vitro studies revealed that HRG caused moderate (∼30%) dilation of cannulated cerebral arterioles and proliferation of cerebrovascular endothelial cells. We conclude that HRG released from clotted blood, while unlikely to contribute to cerebral vasospasm, might provide important vasodilatory or angiogenic stimuli after hemorrhagic stroke.
Keywords: coagulation, microvessels, proteomics
Introduction
Rupture of intracranial aneurysms, which occurs in approximately 2% of the population,1 causes diffuse subarachnoid hemorrhage (SAH). Many SAH patients are affected by delayed ischemic neurologic deficits associated with cerebral vasospasm 5 to 14 days after SAH. Large artery vasospasm and microvascular dysfunction are presumed to restrict blood flow and cause ischemic stroke.2 Numerous causative agents of vasospasm have been suggested, yet the majority of these agents are most abundant immediately after hemorrhage (0 to 24 hours) and thus seem unlikely to cause delayed vasoconstriction (onset at ∼4 days post SAH in humans3). We therefore investigated changes in the proteome of aging blood clots to identify proteins that are released over time and that might be involved in the pathogenesis of cerebral vasospasm and delayed ischemic neurologic deficits.
Materials and Methods
Animals
New Zealand white rabbits (n=3) were sedated with acepromazine (2 mg/kg intramuscular) and blood was collected from the central artery of the ear. Domestic pigs (8 to 12 weeks' old of either sex; 7 to 10 kg, n=6) were sedated with tiletamine/zolazepam (Telazol, 4.4 to 6 mg/kg, intramuscular) and anesthetized with 2% to 4% isoflurane. After a left thoracotomy, the heart was excised for a separate study, then the skull was removed and the brain was carefully isolated and placed in cold (5°C) saline solution. All procedures were approved by the Institutional Animal Care and Use Committee of Texas A&M University and performed at an AALAC accredited facility in accordance with the guidelines established by the National Institutes of Health.
Blood Processing and Culture
Aliquots of clotted rabbit blood were placed in an incubator at 37°C, aged under sterile conditions in the dark for up to 120 hours, and harvested after 0, 24, 72, and 120 hours. Each sample was then centrifuged at 1400 × g and the resulting serum was frozen at −80°C.
Protein Identification
Rabbit serum samples (n=3) were passed through heparin-affinity chromatography columns (HiTrap, GE Healthcare, Uppsala, Sweden) and the eluent was electrophoresed on a 4% to 12% Bis-Tris gradient gel (Invitrogen, Carlsbad, CA, USA) and stained with GelCode Blue (Thermo Scientific, Rockford, IL, USA) to visualize proteins. N-terminus protein sequencing was performed by the Protein Chemistry Laboratory at Texas A&M University on an Applied Biosystems Procise 492 protein sequencer. Desalted chromatography eluants (n=3) as well as serum samples from an additional four rabbits were immunoblotted for histidine-rich glycoprotein (HRG; primary antibody polyclonal mouse anti-human HRG, 1:2,500, Novus Biologicals, Littleton, CO, USA; secondary antibody HRP-conjugated goat anti-mouse IgG Fcγ, 1:10,000, Jackson Immunoresearch, West Grove, PA) using standard protocols.4 Bands were quantified using ImageJ software5 and normalized to total protein concentration as measured by BCA assay (Thermo-Pierce).
Functional Assays
Single porcine pial arteriolar branches (20 to 60 μm in internal diameter in situ, 0.6 to 1.0 mm in length without branches) were isolated from the surface of the cerebral cortex and cannulated for in vitro study utilizing video microscopic techniques as described previously.6 Arteriolar responses to recombinant human HRG (R&D Systems, Minneapolis, MN, USA; reconstituted in physiological saline solution) were recorded. Porcine arterioles were used in an attempt to avoid potential problems resulting from low homology levels between rabbit and human HRG. All arterioles were pressurized to 60 cmH2O intraluminal pressure without flow and developed myogenic tone (54±3% of maximum diameter), constricted in the presence of 1 nM endothelin-1 (60% to 70%), then dilated when exposed to 1 nM bradykinin (80% to –90%). Average resting diameter was 38±6 μm and average maximum diameter was 82±9 μm. At the end of each functional experiment, the vessel was relaxed with 0.1 mmol/L sodium nitroprusside in ethylenediaminetetraacetic acid (1 mmol/L)-Ca2+-free physiological saline solution to obtain its maximum diameter at 60 cmH2O intraluminal pressure. Proliferation assays of murine cerebrovascular endothelial (CVE) cells were performed as described previously7 in the presence or absence of HRG. Cells were cultured for 48 hours, then treated with the live-cell stain MTS (Promega, San Luis Obispo, CA, USA). Absorbance of the lysate was read with a spectrophotometer at 490 nm. Concentrations of HRG used in functional experiments were chosen based on published in vitro data.8, 9, 10 The human HRG protein used herein shows a relatively high degree of homology with predicted sequences of both porcine and murine HRG (64% and 63% homology, respectively).
Cerebrovascular Endothelial Cell Staining and Visualization
To visualize CVEs at the completion of the proliferation assays, CVEs were fixed with 4% paraformaldehyde for 15 minutes, rinsed with PBS, and stained with crystal violet solution (2.3% in 20% ethanol) for 10 minutes, followed by two washes and storage in PBS. Pictures were taken on a Nikon Eclipse Ti phase microscope with an AxioCam MRc5 camera, using Axiovision R 4.8.2 software (Carl Zeiss, Inc., Thornwood, NY, USA).
Data Analysis and Statistics
Data were analyzed using Prism 6.0 software (GraphPad, San Diego, CA, USA). Diameter changes in response to HRG were normalized to the arteriole's maximally dilated diameter and expressed as percent maximum dilation. One-way analysis of variance was used to evaluate the change in HRG abundance in electrophoresed gels and immunoblots.
Results
Isolation of Clot Proteins
The protein profile of serum from aged arterial blood (n=3) was analyzed using heparin-affinity chromatography as many growth factors and clotting proteins, a number of which have been implicated in the development of vasospasm,11 bind to heparin. Several minor proteins were released from the clot during the culture (data not shown); we focused on the band that showed the largest change in relative abundance. Relative to control at time 0, the integrated density of this 70 kDa band was elevated 393% at 24 hours (P=0.02), 280% at 72 hours (P=0.03), and 119% at 120 hours (P=0.19).
Identification of Histidine-Rich Glycoprotein
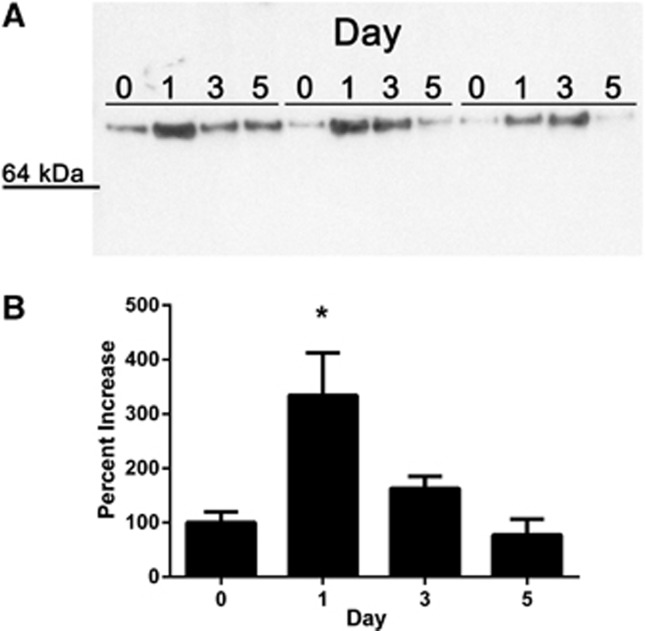
The N-terminal sequence of the protein isolated from the 70 kDa band was LTPTDX, with X most likely representing cysteine. This sequence was compared with the rabbit proteome using both the MASCOT and BLAST search algorithms. Three rabbit proteins contained this amino-acid sequence (LTPTD or LTPTDC) and of these, only HRG was of the correct molecular weight and contained the LTPTDC sequence at the N-terminus. Immunoblotting of the chromatographic eluants confirmed the identity of HRG and showed a significant elevation in protein abundance at 24 and 72 hours of culture (P<0.05), but not at 120 hours (Figure 1). Similar results were seen using whole serum supernatants from aged blood clots (n=4), although these values did not reach statistical significance (data not shown).
Figure 1.
Histidine-rich glycoprotein (HRG) is released from an aging blood clot. Immunoblots confirmed increased HRG protein at 24 and 72 hours of culture (A). Changes in band intensity are shown in the bar graph on the bottom (B). Day of culture is indicated at the top of the blot (0, 1 (24 hours), 3 (72 hours), and 5 (120 hours). Samples from three different animals are shown (animal 1, lanes 1 to 4; animal 2, lanes 5 to 8; animal 3, lanes 9 to 12). Data are presented as mean±standard error of the mean. *Different from day 0, P<0.05.
Vascular Responses to Histidine-Rich Glycoprotein
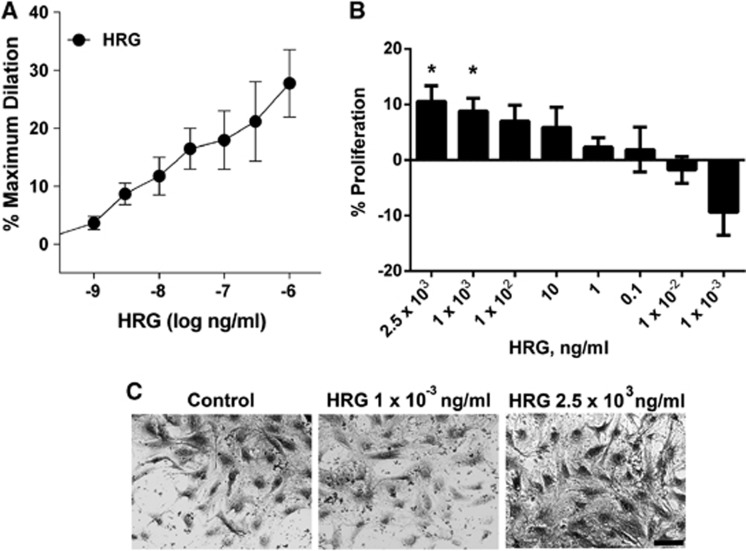
We next assessed cerebral arteriolar responsiveness to HRG. Acute addition of HRG (1 ng/mL to 1 μg/mL) to the adventitial bath of cannulated vessels caused a consistent, sustained (⩾5 minutes) vasodilation in all vessels (Figure 2A), although the duration of the response was not specifically tested. The 1 μg/mL dose of HRG caused the greatest dilation (28±6%). The effects of HRG could be ‘washed out' by rinsing the arteriole with fresh physiologic saline solution. In contrast with previous studies in non-cerebral vessels,9, 10 we found that HRG caused proliferation of CVE cells in a dose-dependent manner (Figure 2B). Low concentrations of HRG (1 pg/mL) tended to decrease proliferation without causing visible toxic effects (Figures 2C, P=0.08), whereas elevated concentrations (>1 μg/mL) enhanced proliferation (P<0.05).
Figure 2.
Dose-response effects of histidine-rich glycoprotein (HRG). (A) Administration of HRG (1 ng/mL to 1 μg/mL) caused dilation of porcine cerebral arterioles (n=6). (B) Treatment with HRG (1 pg/mL to 2.5 μg/mL) for 48 hours caused dose-dependent proliferation of murine cerebrovascular endothelial (CVE) cells (n=5) as normalized to phosphate-buffered saline vehicle-treated control. Data are presented as mean±standard error of the mean. (C) Images of CVE cells after 48-hour treatment as labeled. Scale bar is 20 μm.
Discussion
The prevailing paradigm in vasospasm research is that factors present in the subarachnoid clot act directly on endothelial and smooth muscle cells to cause a transient, entrenched narrowing of one or more arteries, which leads to regional ischemia and neurologic deficits. We, like others,12, 13 hypothesized that the agent(s) responsible for vasospasm might be released from the clot as it ages. In this study, we identified a major protein released from aging blood clots as HRG and observed, for the first time, its vasodilatory effects on cerebral arterioles and proliferative actions on CVE cells.
The vasodilator actions of HRG, in combination with its early (relative to the development of vasospasm) release from clotted blood, make it an unlikely contributor to the vascular dysfunction and cerebral ischemia often seen after SAH. However, it is possible that the release of HRG in the first 72 hours after clot formation could provide a vasodilator stimulus to counteract effects of various vasoconstrictors present at that time. The growth-promoting actions of HRG on CVE cells is also intriguing, given its well-documented anti-angiogenic effects in non-cerebral vessels,8, 9, 10 although the in vivo relevance of this finding remains to be determined. We tested concentrations of HRG within the range of those generally used in cell culture experiments,9 although the concentration of HRG in the cerebrospinal fluid after SAH is likely to be much higher. Based on published measurements of clot volume and rate of clearance,14 we estimate the concentration of HRG in cerebrospinal fluid to be between 10 and 85 μg/mL (∼0.17 to 1.42 μM). It is thus possible that the in vivo actions of HRG are reflective of the higher concentrations tested herein (e.g., vasodilation and cellular proliferation) rather than the anti-angiogenic effects shown previously, although this hypothesis was not specifically tested in the current study. The vascular effects of HRG in combination with its immunomodulatory15 properties make HRG a potentially important player in determining the outcome of delayed cerebral vasospasm and hemorrhagic stroke.
Acknowledgments
We gratefully thank Ms Wenjuan Xu for her technical assistance and Dr Jay Humphrey for his support and critical review of the manuscript.
The authors declare no conflict of interest.
Footnotes
Support: NIH HL-80415, NIH NS-62242.
References
- Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251–256. doi: 10.1161/01.str.29.1.251. [DOI] [PubMed] [Google Scholar]
- Sabri M, Ai J, Lakovic K, Macdonald RL. Mechanisms of microthrombosis and microcirculatory constriction after experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:185–192. doi: 10.1007/978-3-7091-1192-5_35. [DOI] [PubMed] [Google Scholar]
- Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man. J Neurosurg. 1978;48:173–178. doi: 10.3171/jns.1978.48.2.0173. [DOI] [PubMed] [Google Scholar]
- Steelman SM, Chowdhary BP. Plasma proteomics shows an elevation of the anti-inflammatory protein APOA-IV in chronic equine laminitis. BMC Vet Res. 2012;8:179. doi: 10.1186/1746-6148-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS.Imagej. US National Institutes of Health, Bethesda, MD, USA; ; http://imagej.nih.gov/ij/ , 1997–2011.
- Hein TW, Kuo L. Camp-independent dilation of coronary arterioles to adenosine: role of nitric oxide, G proteins, and K(ATP) channels. Circ Res. 1999;85:634–642. doi: 10.1161/01.res.85.7.634. [DOI] [PubMed] [Google Scholar]
- Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005–3023. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixelius J, Olsson AK, Thulin A, Lee C, Johansson I, Claesson-Welsh L. Minimal active domain and mechanism of action of the angiogenesis inhibitor histidine-rich glycoprotein. Cancer Res. 2006;66:2089–2097. doi: 10.1158/0008-5472.CAN-05-2217. [DOI] [PubMed] [Google Scholar]
- Guan X, Juarez JC, Qi X, Shipulina NV, Shaw DE, Morgan WT, et al. Histidine-proline rich glycoprotein (HPRG) binds and transduces anti-angiogenic signals through cell surface tropomyosin on endothelial cells. Thromb Haemost. 2004;92:403–412. doi: 10.1160/TH04-02-0073. [DOI] [PubMed] [Google Scholar]
- Juarez JC, Guan X, Shipulina NV, Plunkett ML, Parry GC, Shaw DE, et al. Histidine-proline-rich glycoprotein has potent antiangiogenic activity mediated through the histidine-proline-rich domain. Cancer Res. 2002;62:5344–5350. [PubMed] [Google Scholar]
- Miller CA, Lombard FW, Wu CT, Hubbard CJ, Silbajoris L, Borel CO, et al. Role of vascular mitogens in subarachnoid hemorrhage-associated cerebral vasculopathy. Neurocrit Care. 2006;5:215–221. doi: 10.1385/NCC:5:3:215. [DOI] [PubMed] [Google Scholar]
- Guan YY, Weir BK, Marton LS, Macdonald RL, Zhang H. Effects of erythrocyte lysate of different incubation times on intracellular free calcium in rat basilar artery smooth-muscle cells. J Neurosurg. 1998;89:1007–1014. doi: 10.3171/jns.1998.89.6.1007. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Handa H, Toda N. Role of intrinsic arachidonate metabolites in the vascular action of erythrocyte breakdown products. Stroke. 1984;15:60–64. doi: 10.1161/01.str.15.1.60. [DOI] [PubMed] [Google Scholar]
- Reilly C, Amidei C, Tolentino J, Jahromi BS, Macdonald RL. Clot volume and clearance rate as independent predictors of vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:255–261. doi: 10.3171/jns.2004.101.2.0255. [DOI] [PubMed] [Google Scholar]
- Gorgani NN, Smith BA, Kono DH, Theofilopoulos AN. Histidine-rich glycoprotein binds to DNA and fc gamma RI and potentiates the ingestion of apoptotic cells by macrophages. J Immunol. 2002;169:4745–4751. doi: 10.4049/jimmunol.169.9.4745. [DOI] [PubMed] [Google Scholar]