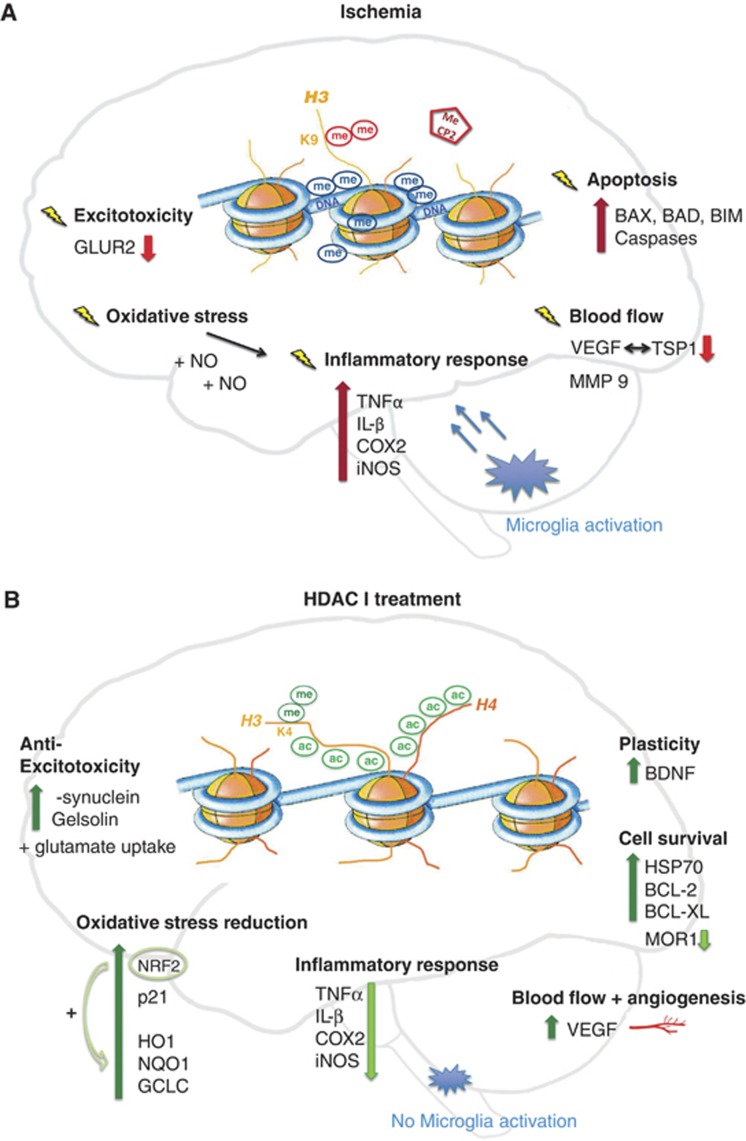
Figure 2.
Ischemic pathways of damage (A) and protection after histone deacetylase inhibitor (HDACi) administration (B). Cerebral ischemia (A) globally leads to transcriptional repression in the cell. This effect is mirrored on the epigenetic level, where a rise in repressive DNA methylation (me) marks is observed together with vast histone 3 (H3) and histone 4 (H4) deacetylation. Additionally, H3 dimethylation at lysine 9 (H3K9me2), a further repressive sign, is enhanced. Further, transcriptional silencers, such as methyl-CpG binding protein 2 (MeCP2), are recruited to repressed gene promoters. Changes in gene regulation occur in most diverse pathways of ischemic disease development such as exitotoxicity, oxidative stress, inflammation, changes in blood flow, as well as cell death, or apoptosis. Examples for repressed genes are the glutamine receptor (GLUR2) in neurons, and thromobospondin1 (TSP1) as well as its angiogenic antagonist vascular endothelial growth factor (VEGF) in different phases of ischemic damage. However, an upregulation of pro-inflammatory and apoptotic players is visible in spite of a global repression. Examples for inducers of inflammation are tumor necrosis factor-α (TNFα), interleukin-β (IL-β), cyclooxygenase 2 (COX2) or the nitric oxide (NO) synthase (iNOS). Upregulated pro-apoptotic candidates are the BCL-2 family members BCL-2-associated X protein (BAX), BCL-2-associated death promoter (BAD) and BCL-2-like protein 11 (BIM) as well as diverse caspases. However, treatment with histone deacetylase inhibitors (HDACi) in ischemia (B) massively alters gene expression. H3 and H4 acetylation levels are maintained and no increase in DNA methylation is visible. Furthermore, activating histone methylation marks, such as H3K4 dimethylation (H3K4me2) accumulate. Many protective genes are actively expressed and modulate the response to ischemia. Upregulated gelsolin and α-synuclein levels are involved in the attenuation of excitoxicity. Protective anti-oxidative response genes are, for example, nuclear factor 2 (NRF2) together with its downstream elements heme oxygenase 1 (HO1), NADPH dehydrogenase quinone 1 (NQO1) and glutamate cysteine ligase (GCLC). An extended upregulation of the angiogenic factor VEGF modulates blood flow. Moreover cell survival pathways are boosted: important mediators are BCL-2 BCL-XL and the chaperone HSP70. Regenerative pathways are induced with the help of brain-derived neurotrophic factor (BDNF). To support pro-survival signaling, genes involved in ischemic cell death such as opioid receptor (MOR1) are repressed as well as inflammatory pathways.