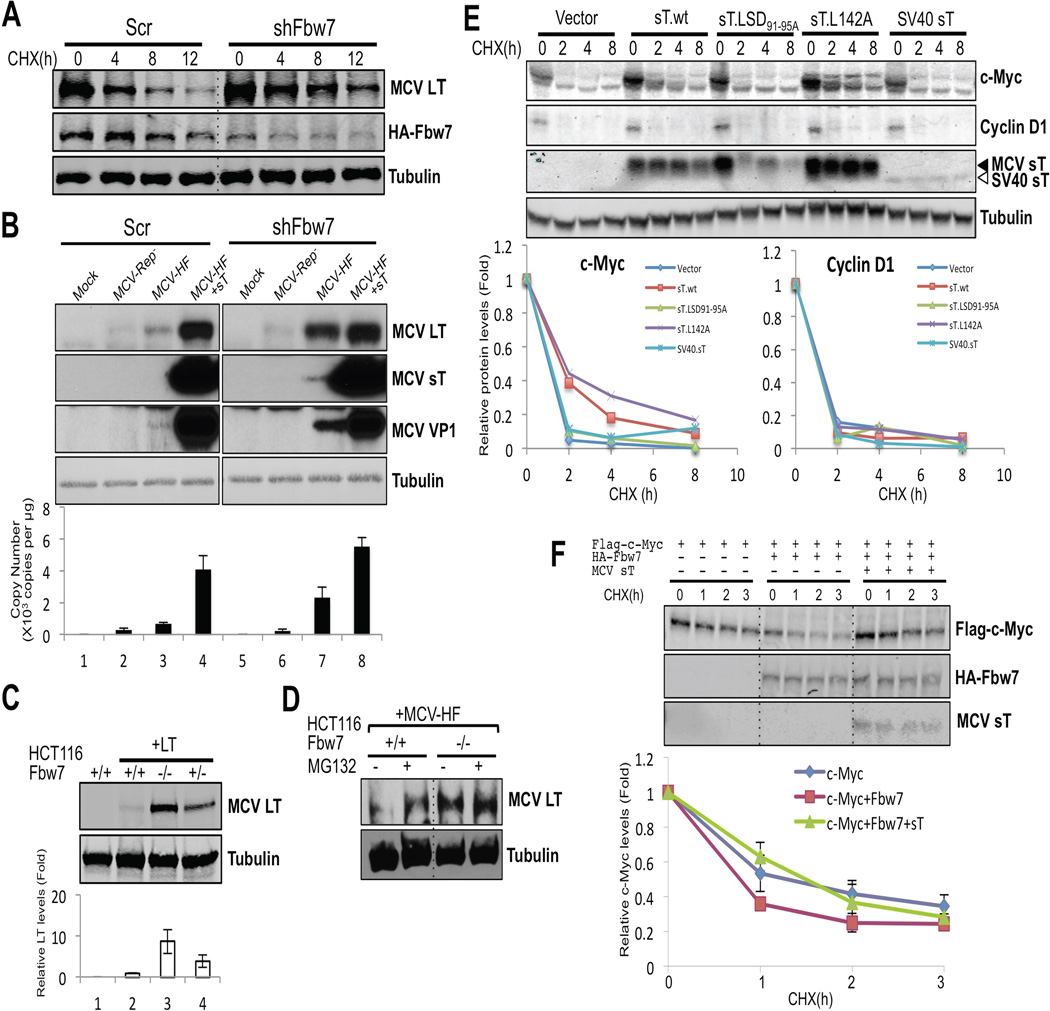
Figure 5. SCFFbw7 inhibits MCV replication and increases turnover of viral and cellular oncoproteins.
(A) Knockdown of Fbw7 reduces LT protein turnover. HA-Fbw7 and LT were cotransfected together with either scrambled shRNA (Scr) or Fbw7 shRNA (shFbw7.2) constructs into 293 cells. 3 days after transfection cells were treated with CHX, and LT and Fbw7 protein expressions were measured by immunoblotting. (B) Knockdown of Fbw7 promotes MCV replication and protein expression. MCV genomic clone early and late protein expression (top panels) and genome copy number (bottom panel) are increased in 293 cells transduced with Fbw7 shRNA compared to scrambled shRNA. MCV-Rep− is a replication defective clone of MCV-HF having a point mutation in its replication origin. Each cell line was mock transfected or transfected with a genomic clone for 5 days prior to harvesting. In lanes 4 and 8, an sT expression vector was cotransfected in trans with MCV-HF. Replication efficiency was measured in triplicate by qPCR. Error bars represent SEM; n = 3. (C) Genetic deletion of Fbw7 increases ectopic LT expression in HCT116 cells. LT expression was examined by immunoblotting with CM2B4 antibody in HCT116 cells null (−/−), heterozygous (+/−) or wild-type (+/+) for the Fbw7 gene. Quantification was performed with LI-COR. Error bars represent SEM; n = 3. (D) Knockout of Fbw7 decreases proteasomal degradation of MCV LT from MCV-HF virus. MCV-HF was transfected into HCT116 wild-type and Fbw7-null cells. 5 days after transfection, cells were treated with MG132 or DMSO for 12 h and LT protein levels were measured by immunoblotting. In wild-type cells, virus-generated LT protein was increased by proteasome inhibition. In Fbw7 null cells, LT protein expression was elevated and unchanged by MG132 treatment. (E) sT stabilizes a proto-oncogene c-Myc but not cyclin D1 in Rat-1 cells. Rat-1 cells were stably transduced with vector, wild type sT, sT.LSD91-95A, L142A and SV40 sT and turnover of c-Myc and cyclin D1 were measured by CHX treatment. c-Myc protein was stabilized by either MCV sT.wt or sT.L142A but not by sT.LSD91-95A or SV40 sT. MCV sT did not affect turnover of cyclin D1. Mixture of CM8E6 and pAb419 antibodies was used to detect viral sTs. (F) sT decreases the degradation rate of c-Myc. Flag-c-Myc expression construct was cotransfected with either empty vector or HA-Fbw7, with or without sT, and turnover was measured in a CHX chase assay. Fbw7 overexpression destabilized exogenous c-Myc protein and this was reversed by coexpression of MCV sT. Error bars represent SEM; n = 3. See also Figure S4.