FIGURE 1.
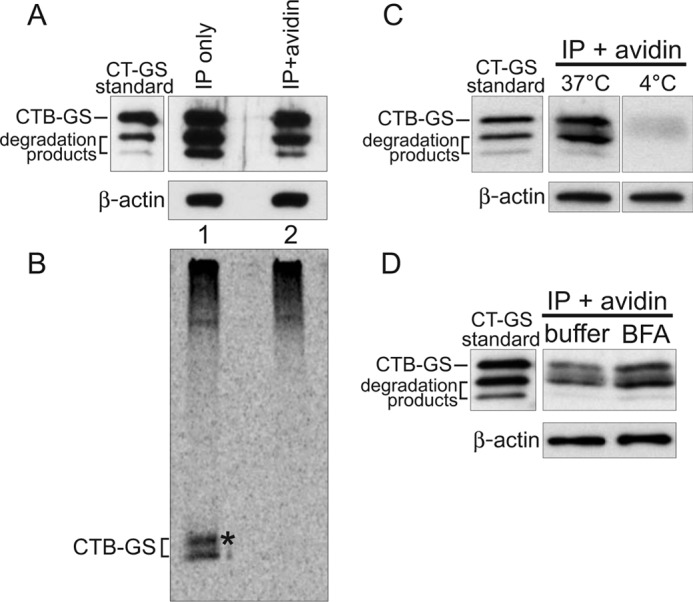
Sorting of CT into the transcytotic pathway bypasses retrograde transport to the TGN and ER. A–D, polarized T84 monolayers were incubated apically with 10 nm CT-GS for 2 h at 37 °C prior to biotinylation of the basolateral surfaces. Total toxin was immunoprecipitated from cell lysates and either analyzed directly (IP only, captures all CT-GS) or subjected to an additional avidin affinity purification step (IP + avidin, captures only basolateral CT-GS). To achieve similar CTB-GS levels between the total and basolateral pools, 7-fold more cells were used for the IP + avidin samples. 10% of each sample was analyzed by SDS-PAGE and immunoblot analysis using anti-CTB (A), with the remainder being separated by SDS-PAGE and analyzed by autoradiography (B). Pure CT-GS is indicated (standard), and crude lysates were separated by SDS-PAGE and probed with an antibody against β-actin as a loading control (A, C, and D, lower panels). The asterisk in B denotes a higher molecular weight glycosylated CTB-GS band. C, T84 monolayers were treated as in A, except cells were not pretreated with 35S-sulfate, and some cells were held at 4 °C during the 2-h apical toxin incubation. Basolateral CT-GS fractions (IP + avidin) were immunoblotted using anti-CTB. D, as in C, except cells were incubated with either buffer or 10 μm BFA for 20 min prior to apical exposure to 10 nm CT-GS for 2 h at 37 °C. Data are representative of three independent experiments for A–C and two independent experiments for D.
