FIGURE 5.
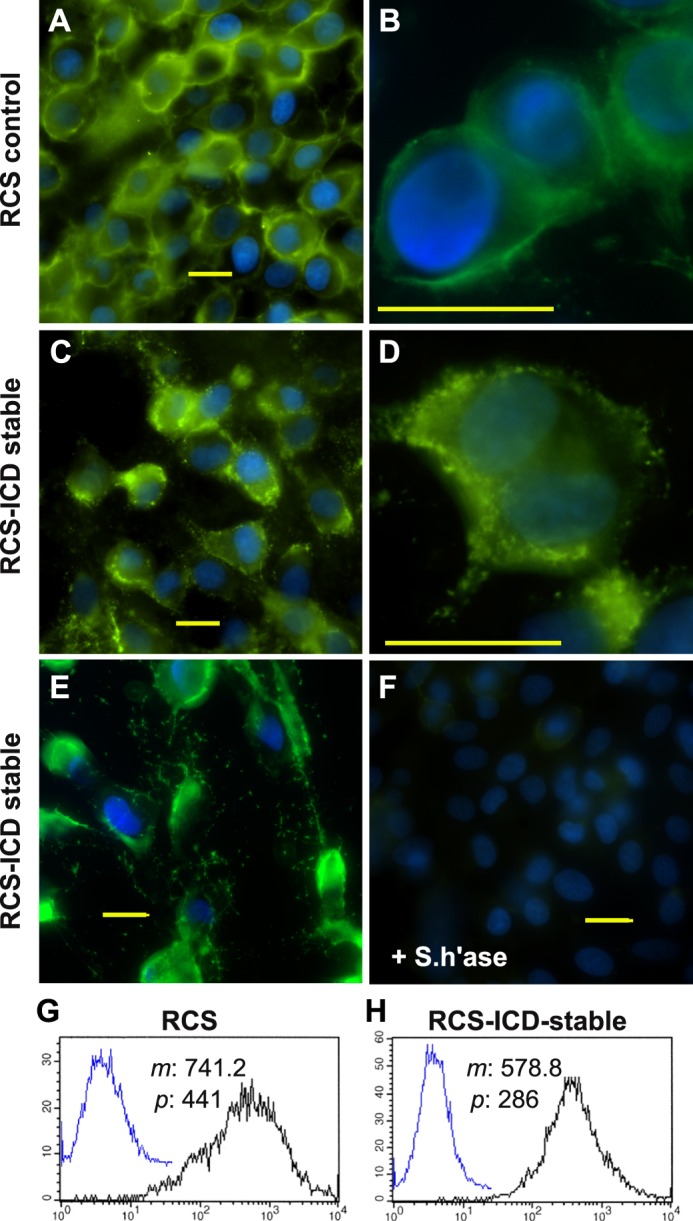
Cell surface retention of endogenous and exogenous hyaluronan on RCS cells. Control, parental RCS cells (panels A and B) were compared with CD44-ICD stable transfectants of RCS (RCS-ICD stable, panels C–E) for retention of hyaluronan at the cell surface. Cells grown on coverslips were untreated (panels A–E) or pretreated with 5 units/ml Streptomyces hyaluronidase (+S.h'ase) for 3 h at 37 °C (panel F). The washed cells were then fixed and stained for hyaluronan using biotinylated hyaluronan-binding protein followed by neutravidin-FITC, mounted in medium containing DAPI, and visualized by fluorescence microscopy. Shown are digital overlay images of green (hyaluronan) and blue (nucleus) fluorescence channels. Bars indicate 20 μm. Flow cytometric analysis was used to compare the binding of exogenous hyaluronan to control RCS cells (panel G) and CD44-ICD stable transfectants of RCS (panel H). High density monolayers of each cell type were pretreated with testicular hyaluronidase to remove endogenous matrix. The cells were next brought into suspension using non-enzymatic dissociation solution and then washed. One group of washed cells was left untreated to serve as controls (panels G and H, overlay blue line tracing). Another group of cells was incubated with 60 μg/ml fluorescein-conjugated hyaluronan (panels G and H, black line). Cell surface immunofluorescence was quantified using a FACScanTM cytometer; mean channel fluorescence (m) and peak channel fluorescence (p) values are included. Cells preincubated with unlabeled hyaluronan before the addition of fluorescein-conjugated hyaluronan exhibited substantially reduced mean channel fluorescence (data not shown).
