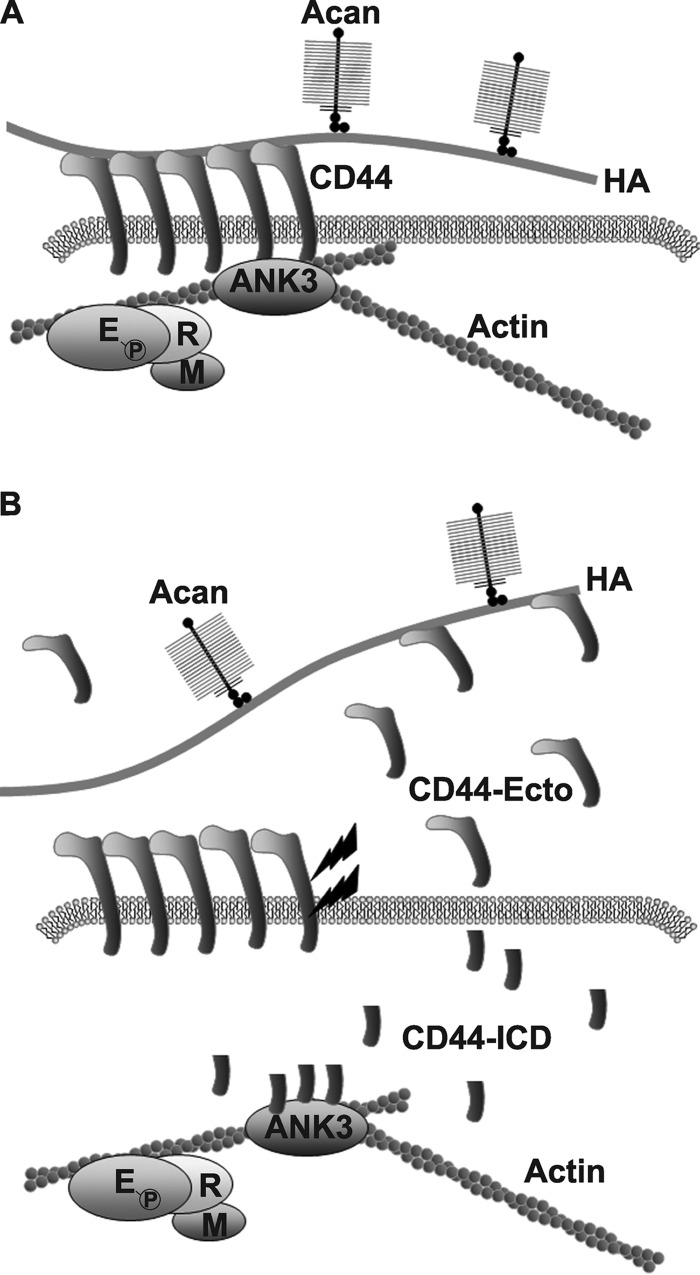
FIGURE 8.
A proposed model for CD44-ICD mediated interference with the interaction of full-length CD44 with actin adaptor proteins in chondrocytes. The chondrocyte pericellular matrix is anchored to the cell surface in large part by the binding of aggrecan (Acan)/link protein/hyaluronan (HA) complexes to CD44 (panel A). CD44 in turn is anchored to the intracellular cortical actin cytoskeleton via interaction with the adaptor protein, ankyrin 3 (ANK3). Members of the ERM adaptor proteins are also present within the complex. When CD44 undergoes proteolytic cleavage (lightning bolts), fragmented products are released including a remnant CD44 extracellular domain (CD44-Ecto) and an intracellular CD44 domain (CD44-ICD) (panel B). Whether by natural cleavage or overexpression of a recombinant transgene, CD44-ICD competes with the binding of full-length CD44 with ANK3. This interference by CD44-ICD results in blockage of binding of the remaining full-length CD44 (receptors that would be otherwise functional) with the underlying actin cytoskeleton. Under these conditions, the capacity of CD44FL to bind extracellular HA is reduced, and the CD44 is more readily solubilized from the membranes with lower Igepal detergent conditions. As such, the CD44-ICD could be considered a dominant-negative agent, producing an inside-out effect on coat retention.