FIGURE 4.
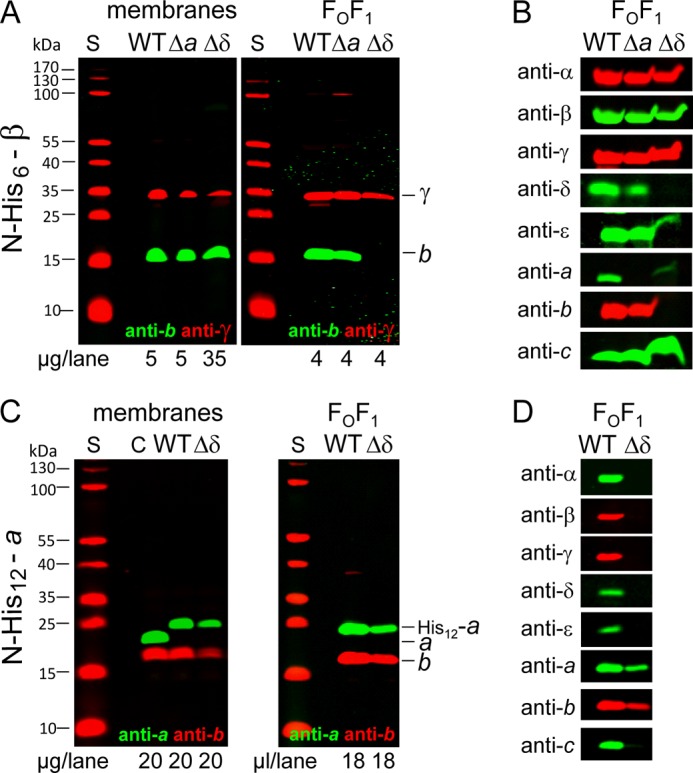
Purification of partially assembled FOF1 of mutants Δa and Δδ by affinity chromatography in comparison with wild type. A and C, comparison of membranes and purified FOF1 complexes. B and D, detection of the different FOF1 subunits to verify their presence in the complexes purified. DK8 transformed with pBWU13 derivatives was grown as described in the legend of Fig. 2. Membranes were prepared and solubilized, and FOF1 complexes were purified by affinity chromatography via a His6 tag (A and B) present N-terminal to subunit β (WT, pBH7; Δa, pBH55.β-His; Δδ, pBH56.β-His) or a His12 tag (C and D) fused to the N terminus of subunit a (WT, pBH1; Δδ, pBH56.a-His12). Membranes and purified FOF1 were analyzed by immunoblotting. S, molecular mass standard; C, subunits a and b detected in membranes of DK8/pBWU13.
