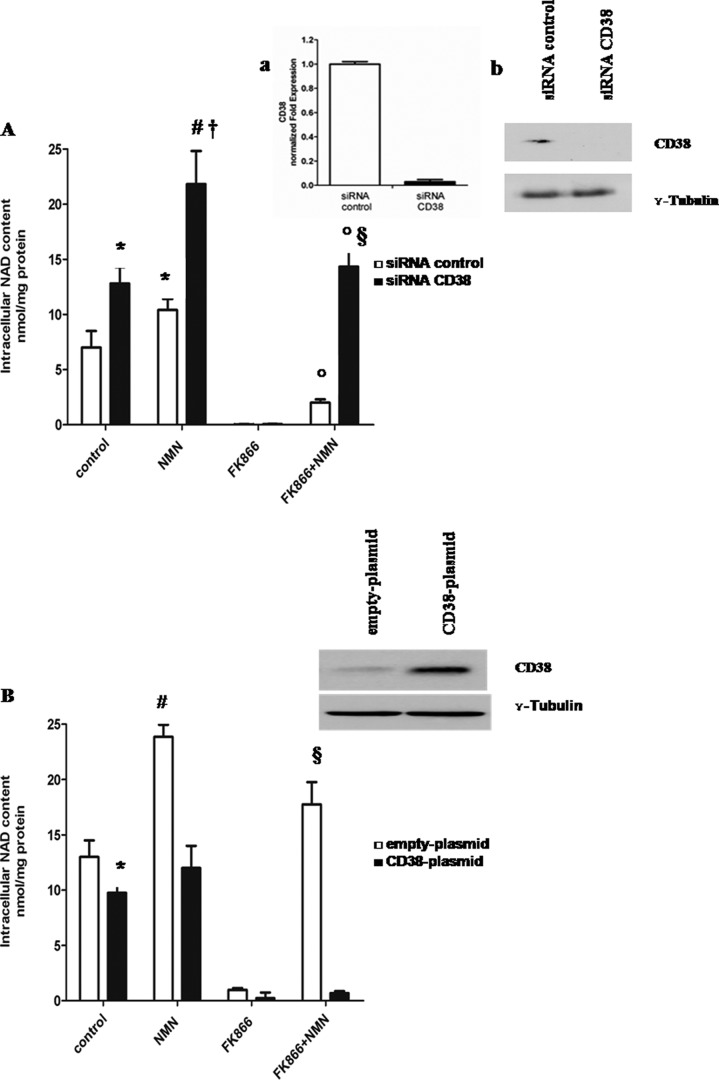
FIGURE 3.
CD38 expression affects intracellular NAD+ synthesis triggered by extracellular NMN. A, A549 cells were transfected with specific siRNA for CD38 or with negative control siRNA (siRNA control) and seeded in 12-well plates (2 × 105 cells/well). Cells were treated for 72 h with or without 30 nm FK866 in the presence or absence of 10 μm NMN (added twice a day). Cells were harvested and lysed in 0.6 m PCA, and NAD+ content was measured in neutralized extracts. NAD+ values were normalized to protein content. Data are expressed as mean ± S.D. (error bars) (n = 3). *, p < 0.05; #, p < 0.01 compared with untreated siRNA control cells; †, p < 0.01 compared with untreated siRNA CD38 cells; °, p < 0.001 compared with FK866-treated cells; §, p < 0.001 compared with FK866 + NMN-treated siRNA control cells. Inset, 24 h after transfection; a, qPCR analysis was performed, and results are normalized on the reference genes GAPDH and HPRT1 and compared with negative control; b, Western blot analysis of CD38 protein level was performed (results from one representative experiment is shown). B, U87 cells were transfected in parallel with pcDNA3.1 (empty plasmid) or with CD38-pcDNA3.1 (CD38 plasmid) using the Nucleofector system and seeded in 12-well plates (2 × 105 cells/well). Cells were then treated for 72 h with or without 30 nm FK866 in the presence or absence of 10 μm NMN (added twice a day). Cells were harvested and lysed in 0.6 m PCA, and NAD+ content was measured in neutralized extracts. NAD+ values were normalized to protein content. Data are expressed as mean ± S.D. (n = 3). *, p < 0.05; #, p < 0.01 compared with untreated, empty plasmid cells; §, p < 0.001 compared with FK866-treated empty plasmid cells and with FK866 + NMN-treated CD38 plasmid cells. Inset, 24 h after transfection, Western blot analysis of CD38 protein level was performed (one representative experiment of three comparable ones is shown).