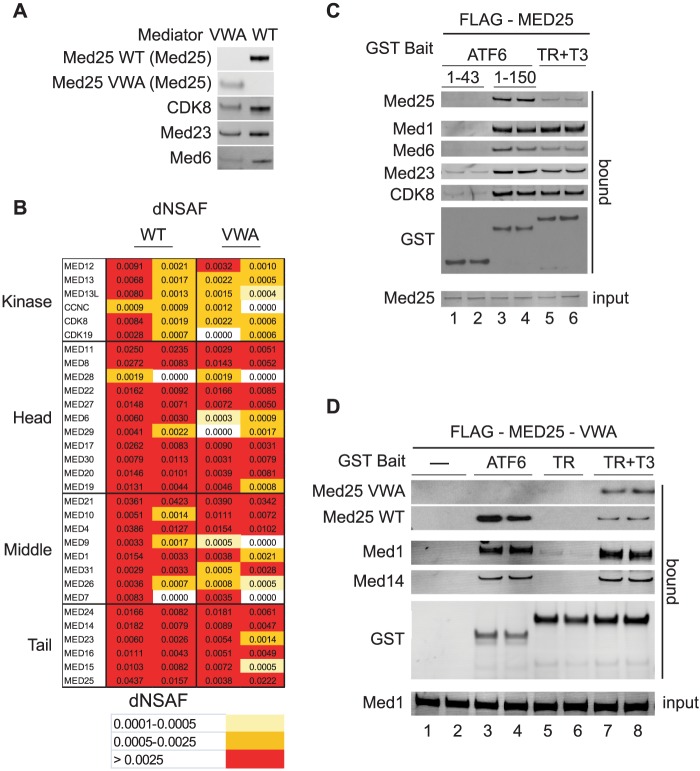
FIGURE 2.
The MED25 VWA domain directs MED25 incorporation into Mediator but does not bind the ATF6α AD. A, full-length FLAG-MED25- or FLAG-MED25-VWA-associated proteins were purified from cells stably expressing them by anti-FLAG-agarose chromatography (20). Western blot analyses of FLAG-MED25- and FLAG-MED25-VWA-associated proteins. The antibodies used are indicated in the figure. B, analyses of FLAG-MED25- and FLAG-MED25-VWA-associated proteins by MudPIT mass spectrometry. The distributed Normalized Spectral Abundance Factor (dNSAF) reflects the relative abundance of the different Mediator subunits in the samples (24). Two independent preparations of FLAG-MED25- and FLAG-MED25-VWA-associated proteins were analyzed. C, glutathione-Sepharose purification of Mediator from nuclear extracts of cells stably expressing FLAG-MED25. Binding reactions contained 20 μl of nuclear extract and 12 pmol of either GST-ATF6α-(1–150), GST-ATF6α-(1–43), or liganded GST-TR (supplemented with 1 μm thyroid hormone T3). Input (10% of total) and bound proteins were detected by Western blotting with the indicated antibodies. D, glutathione-Sepharose purification of Mediator from 100 μl nuclear extract of cells stably expressing FLAG-MED25-VWA, using 12 pmol of GST-ATF6α-AD or liganded GST-TR. Bound Mediator subunits were detected by Western blotting with the indicated antibodies.