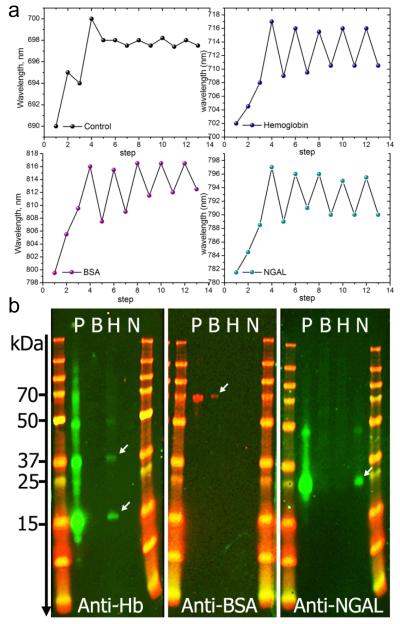
Figure 5.
Reproducibility and selectivity of the MIP-AuNR nanosensors. a) Shift of the LSPR wavelength following the different steps of the imprinting process. Each measurement point represents the shift obtained at the end of each step indicated with numbers. Steps 1 to 4 correspnond to AuNR, AuNR-pATP/GA, AuNR-pATP/GA-protein and AuNR-pATP/GA-protein-siloxane copolymer, respectively. At step 3, the control was exposed to PBS buffer solution without proteins. Steps 5 to 13 correspond to 4 cycles of protein capture and release, resulting in a red-shift or blue-shift, respectively. The same procedure is applied to hemoglobin, BSA and NGAL biomarker. The control was not treated with proteins. b) Western blotting of the elute solutions obtained from molecularly imprinted nanorod substrate prepared with 3 different protein templates: Bovine Hemoglobin (Hb), Bovine Serum Albumin (BSA) and recombinant human NGAL biomarkers. Each panel contains two molecular weight marker columns flanking 4 migration lanes: P is the protein mixture (containing Hb, BSA and NGAL) applied to all prepared MIP-AuNR substrates. Lines B, H and N contain the elute solutions obtained from the MIP-AuNR substrates imprinted separately with BSA, Hb and NGAL respectively. The treatment with the antibody Anti-Hb, reveals 2 clearly identified bands in the H lane indicating the presence of denaturated Hb with a single sub-unit (~ 17 kDa) or two sub-units (~ 37 kDa). A weak band at ~ 50 kDa indicates the presence of a 3 sub-units Hb. The second panel treated with anti-BSA reveals a weak band at ~70 kDa demonstrating the presence of BSA. The anti-NGAL treatment of the last paned shows a clear band at ~ 25 kDa, corresponding to NGAL molecular weight. A weak band is also observed at ~ 45 kDa indicating the presence of NGAL concatemers due to a small amount of recombinant NGAL resulting from plasmid concatemerization.