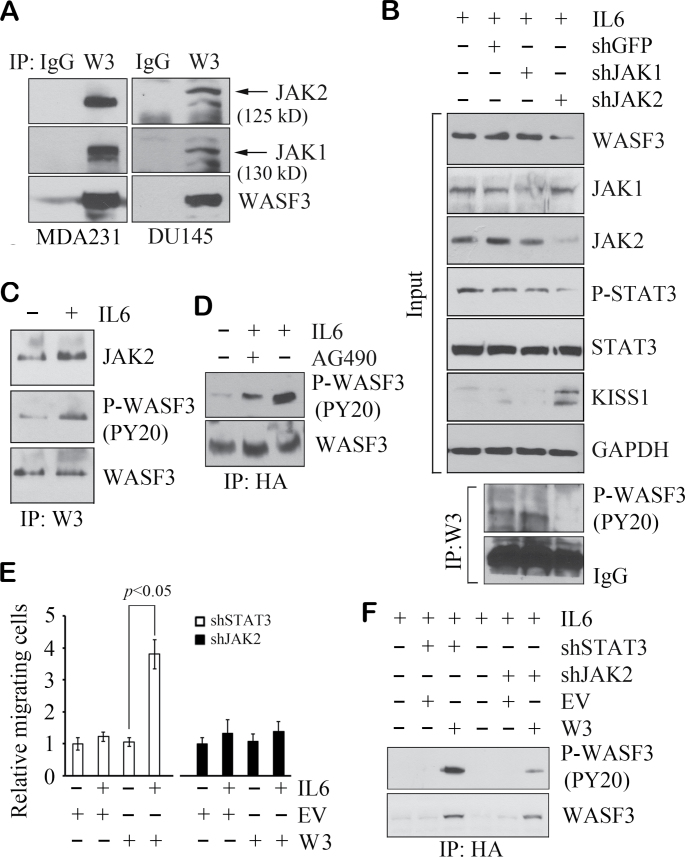
Fig. 4.
JAK2 binds to, and specifically activates, WASF3. IP analysis demonstrates the presence of endogenous JAK1 and JAK2 in WASF3 immunoprecipitates from MDA231 and DU145 cell lysates (A). In MDA231 cells, WASF3 is remarkably suppressed by knockdown of JAK2 but not by loss of JAK1 (B, above). This is reflected in phosphoactivation of WASF3 (B, below). Reduced WASF3 levels correlate with increased KISS1 levels. In (C), IP analysis shows treatment with IL6 for 2 min leads to increased activation of WASF3 and the interaction between JAK2 and WASF3. Starved MCF7 cells forced to express an HA-tagged WASF3 protein show low levels of activated WASF3, but when stimulated with IL6, a dramatic increase in activated WASF3 levels is seen (D). Inactivation of JAK2 in these IL6-treated cells with AG490 leads to reduced levels of activated WASF3 (D). In the MCF7 cells expressing exogenous WASF3 (W3), when STAT3 is knocked down by shRNA, IL6 treatment leads to a significant increase in cell migration (E) compared with untreated cells and cells in which transfected the empty vector (EV). When JAK2 is knocked down in these cells, even in the presence of exogenous WASF3, no difference in migration was detected (E). Data are shown as mean ± SD from three independent experiments (P < 0.05). Analysis of activated WASF3 in these cells shows robust levels in the absence of STAT3 but greatly reduced levels in the absence of JAK2 (F).