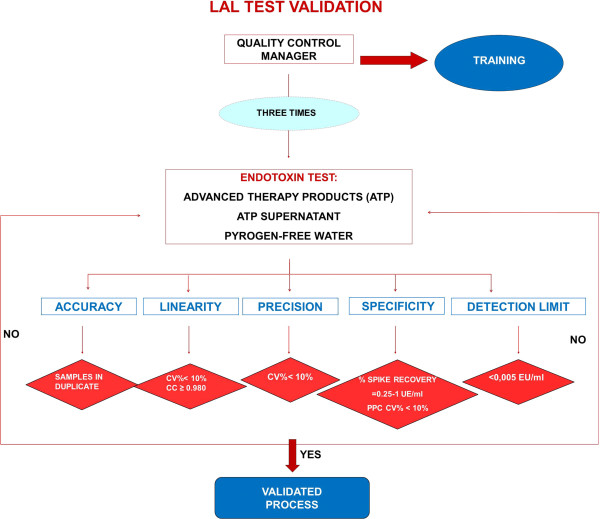
Figure 1.
LAL test validation protocol flow-chart. The test was performed three times under the same operating conditions by the QC manager on the same samples (CTPs, CTPs supernatant, pyrogen-free water) to test precision. According to ICH Q2 we evaluated specificity and the detection limit. To evaluate accuracy, the assay includes seeding each sample in duplicate. For linearity, a standard curve with 0.005 endotoxin unit EU/mL was used. The acceptance criteria were: spike recovery between 0.25 EU/ml – 1 EU/ml with a CV% < 10, standard curve with CV < 10% and correlation coefficient ≥ 0.980.