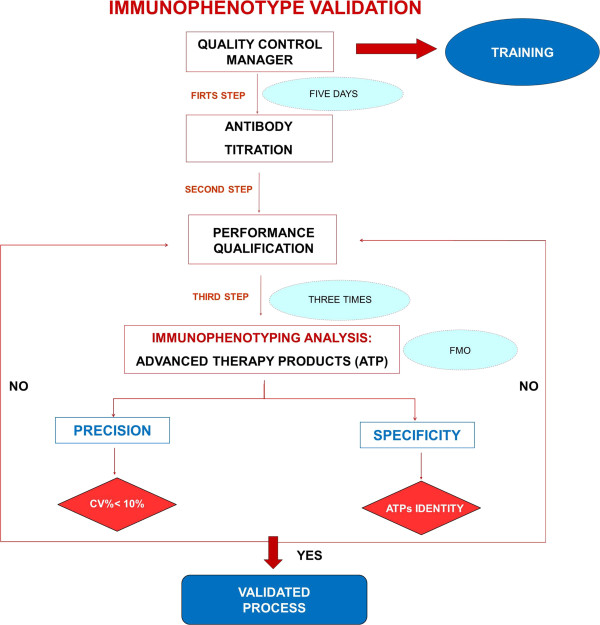
Figure 2.
Immunophenotype validation protocol flow-chart. The immunophenotype validation protocol required: a first step which is the titration of each antibody performed by using scalar antibody dilution; a second step, named Performance Qualification (PQ), during which the QC manager used two types of standard beads to check cytometer reproducibly over time. Immunophenotyping analysis is an identity test to evaluate specificity by using FMO method. The test was performed three times to test precision. The acceptance criteria were: inter-experiment CV% ≤ 10%, BM MSCs positive for CD90, CD73, CD105 and negative for CD45, CD14, CD34, CD19 and HLADR; mDCs positive for CD80, CD86, CD83, CD40, CD11c and HLADR at a high level; CTLs positive for CD3+, CD3 + CD4+, CD3 + CD8+, CD56 + CD3- at a low level and negative for CD19.