Figure 4. Trial sequential analysis of 8 trials assessing vitamin A in a dose at or below RDA (≤ 800 µg) daily versus placebo.
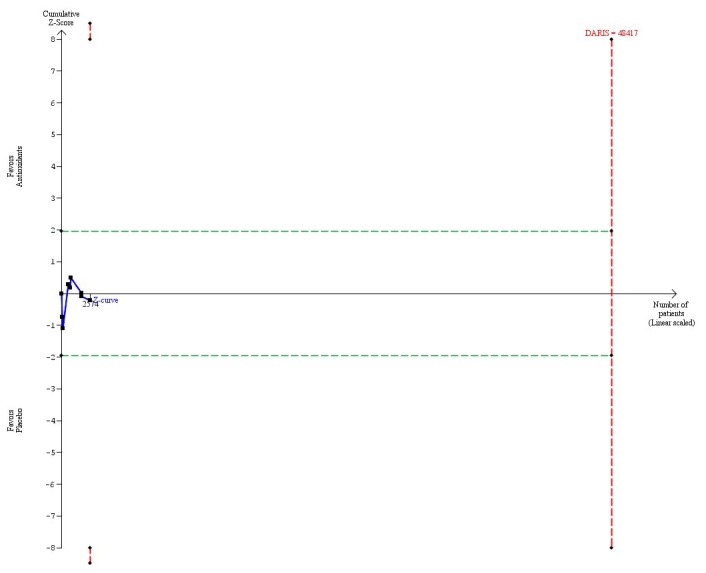
The diversity-adjusted required information size (DARIS = 48,417 participants) was based on a proportion of deaths of 10% in the placebo group; a relative risk reduction of 15% in the vitamin A group; an alpha of 5%; a beta of 20%; and a diversity of 76%. The blue line represents the cumulative Z-score of the meta-analysis. The green lines represent the conventional statistical boundaries. The red lines represent the truncated trial sequential monitoring boundaries. Had we used a relative risk reduction of 5% as planned, the DARIS would have been 456,748 participants and the program could not have drawn the trial sequential analysis due to the fact that the number of randomized patients out of the DARIS is too small. This is why, we post hoc decided to construct the trial sequential analysis with a larger relative risk reduction.
