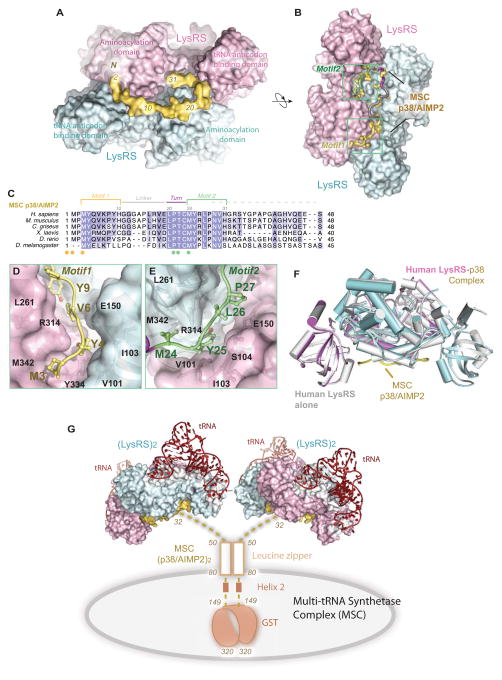
Figure 1. Structural basis for Reserving LysRS in the MSCdirect.
(A–B) Two orthogonal views of the human LysRS:p38/AIMP2 complex structure. Sequence of p38/AIMP2 is color-coded as in (C).
(C) Sequence alignment of the N-terminus of p38/AIMP2 from Drosophila to human. Motifs are colored as Motif 1 (yellow), Gly linker (gray), turn (red) and Motif 2 (green). Spheres represent two similar sets of residues (yellow/green) implicated in the LysRS:p38/AIMP2 interaction.
(D–E) The interface of the LysRS-p38/AIMP2 complex. The surface representation of LysRS is shown. D, Motif 1 of p38/AIMP2 and its interaction with the bottom groove of LysRS. E, Motif 2 of p38/AIMP2 and its interaction with the symmetric groove on the LysRS dimer.
(F) The functional LysRS-p38/AIMP2 complex. Side view of free human LysRS structure (pdb: 3bju, gray) superimposed onto human LysRS:p38/AIMP2 complex, showing the close similarity of LysRS structures with and without binding to p38/AIMP2.
(G) Model of the human multi-tRNA synthetase complex. Dimerization of p38/AIMP2 is through the two helical regions and a C-terminal GST-domain outside of the LysRS binding site. The N-terminal linker (residues 32–48) is disordered as found in the crystal structure. p38/AIMP2 binds to at least 8 of the 11 members of the MSC and forms the core of the MSC. This model represents the canonical functional state of LysRS in the MSC for charging tRNA.
See also Figure S1