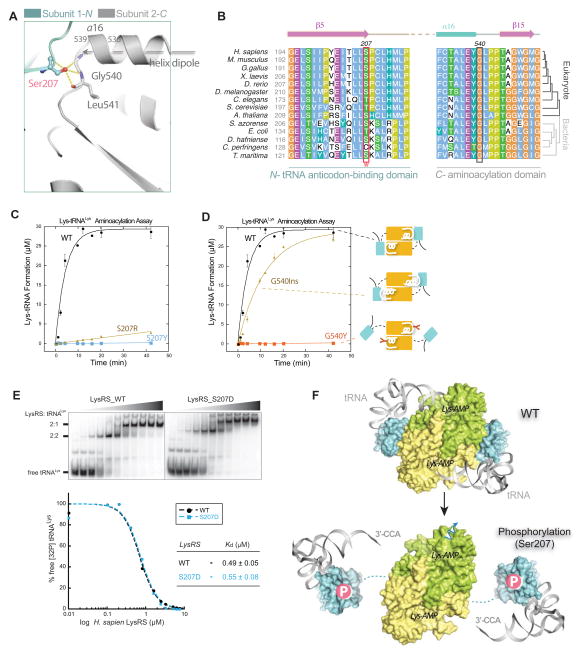
Figure 5. Ser207 as a tipping point for turning off translational function of LysRS.
(A) Close-up view of the domain-domain interface in the human LysRS dimer.
(B) Sequence alignment of the LysRS anticodon-binding domain (N-) and the aminoacylation domain (C-) interface. Phosphorylation site Ser207 and the opposite side G540 mutations analyzed in (C, D) are highlighted in boxes.
(C–D) Aminoacylation assays show that mutations at the dimer interface affect tRNA charging. Mutations with positive charge (S207R) are less effective at inhibiting aminoacylation activity, suggesting that the negative charge of the phosphate group on LysRS_S207 is also important for introducing repulsion between the domain interfaces.
(E) Electrophoretic mobility shift assay shows that LysRSS207D binds tRNA with an affinity similar to that of LysRSWT. Increasing concentrations of the protein (0, 0.1, 0.2, 0.4, 0.8, 1.6, 2.4, 3.2, 5.0, 6.25, and 7.5 μM, respectively) were used.
(F) Mechanism that Ser207-phosphorylation switches off the aminoacylation function of LysRS. Top, a docking model of human LysRS in complex with tRNA by superimposition of the human LysRS structure (pdb3bju) with the yeast AspRS-tRNAAsp complex structure (pdb1asy) shows that the “closed” form of wild type LysRS places the 3′-CCA end of tRNA into the catalytic site. Bottom, the N-terminal tRNA anticodon-binding domain of human LysRSp207 is modeled on the SAXS envelope of LysRSS207D..
See also Figure S5