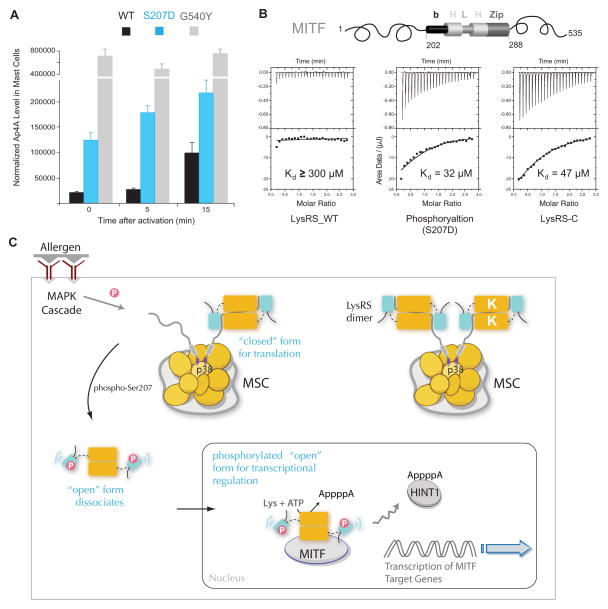
Figure 6. Structural opening directs LysRS to produce Ap4A and bind MITF in cells.
(A) In vivo Ap4A assay shows that the structural-opening mutant LysRSG540Y produced high level of Ap4A independent of mast cell activation. RBL cells were transfected with siLysRS and with plasmids expressing knockdown-resistant versions of LysRSWT, LysRSS207D or LysRSG540Y. After 24 h, cells were stimulated with the IgE-DNP trigger. The means and standard errors of the means for three experiments are shown. P<0.05.
(B) Isothermal titration calorimetry assays for binding of the MITF bHLH-Zip domain to LysRSWT, LysRSS207D or the C-terminal aminoacylation domain of LysRS.
(C) Model for the phosphorylation-dependent translation and transcription switch of LysRS driven by structural opening. In quiescent mast cells, LysRS is associated with p38 in a closed form and is retained in the cytoplasmic MSC. Antigen activation phosphorylates Ser207 and triggers an open form of LysRS. By opening up the structure, phosphorylated LysRS is released from the MSC, translocates from cytoplasm to the nucleus, binds to MITF and generates Ap4A to activate MITF transcription functions. Thus, by selecting two distinct conformers, phosphorylation could switch the functional of LysRS between translation and transcription.
See also Figure S6