Abstract
The acute phase of influenza infection is rarely associated with significant cognitive dysfunction. We describe a case of a 24 year-old man who developed global amnesia in the acute phase of influenza A infection. His deficits resolved over the course of several weeks. Transient abnormalities of diffusion and T2-weighted imaging were seen in the bilateral hippocampi. We review cerebral complications of influenza and discuss the possible role of previously proposed mechanisms in our patient’s case.
Introduction
Influenza A infection typically manifests as a self-limited respiratory and constitutional syndrome without nervous system involvement; however, it is occasionally associated with cerebral manifestations ranging from mild cognitive effects to encephalopathy, parkinsonism, and seizure (Henry, 2010). Here we report a case of a patient who developed an amnestic syndrome associated with T2 abnormalities in the bilateral hippocampi during the acute phase of influenza A infection.
Patient Report
A 24 year-old previously healthy right-handed software engineer developed fever, chills, malaise, diffuse arthralgias and myalgias. The following day, his girlfriend and his sister reported that he was unable to recall concurrent events or events from the past six months. He recognized them, but he did not remember that his sister had recently moved from overseas to San Francisco (where he lives). Despite their attempts to explain, he repeatedly asked where he was, how he got there, and why his sister was there. They brought him to the emergency room for evaluation three days after onset of fever because he was not improving.
Upon arrival at the emergency room, he was found to be afebrile and with normal vital signs. His general exam was unremarkable. He was not agitated, and denied delusions, hallucinations, or significant mood changes. On a brief cognitive test, he was unable to recall any of the three cue words given after a delay of three minutes. The remainder of his neurologic examination was normal. His family confirmed that he had no significant past medical history. There was no known personal or familial history of migraine, seizure, or stroke, and no recent history of head trauma, emotional distress, or strenuous physical activity. He had not developed any febrile illness for the past year and had not travelled out of the country in over six months. He graduated from a four-year college and, at the time of presentation, was employed as a software engineer. Family history included late-onset dementia in his paternal grandfather. Comprehensive laboratory evaluation revealed only a nasal swab positive for Influenza A (Table 1). Non-contrast computed tomography (CT) of the head and a chest radiograph were normal. He was discharged from the emergency room with close outpatient follow up.
Table 1.
Laboratory evaluations
| Day* | Site | Test | Findings |
|---|---|---|---|
| 3 | Nasal swab | Influenza A antigen DFA | Positive |
| CSF | Protein | 18 mg/dL | |
| Glucose | 65 mg/dL | ||
| WBC | 1/mm3 | ||
| RBC | 1/mm3 | ||
| Gram stain | Negative/Normal | ||
| Culture | |||
| HSV PCR | |||
| Serum | Comprehensive Metabolic Panel | ||
| Complete blood count | |||
| Urine | Toxicology | ||
| Urinalysis | |||
| 7 | Serum | RPR | |
| HIV antibody screen | |||
| TSH | |||
| B12 | |||
| ANA |
Days after onset of fever
Abbreviations: DFA, direct fluorescent antibody
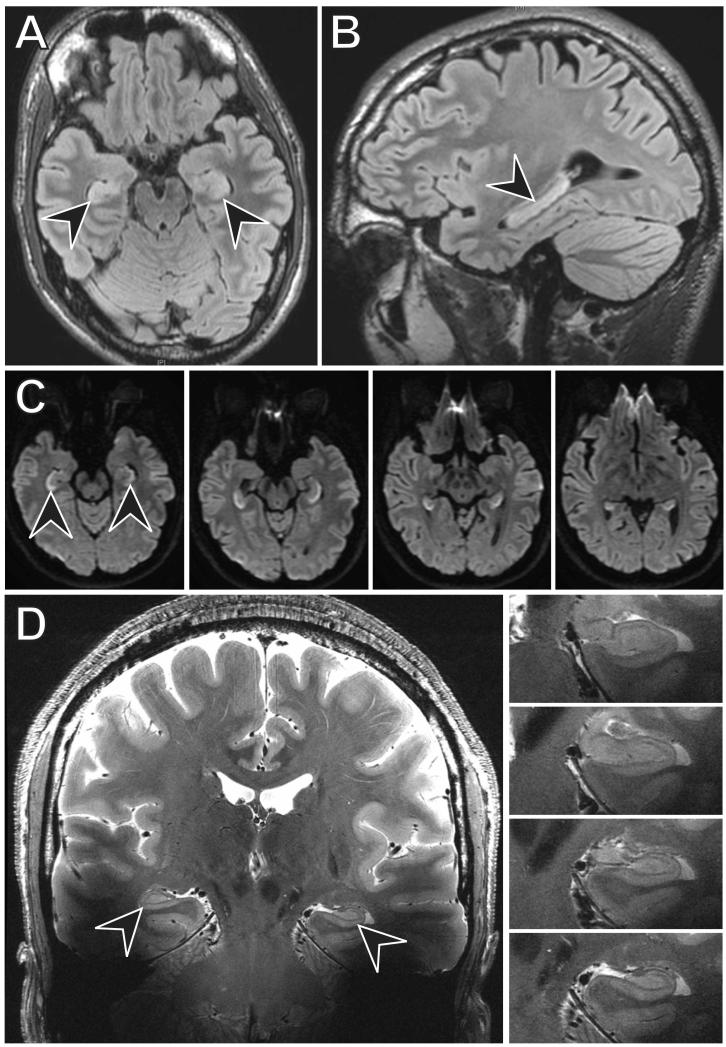
Seven days after symptom onset, the patient was seen in follow-up. At that time, according to the patient and his family, his constitutional symptoms had resolved. His memory was improving but was not at baseline. His Montreal Cognitive Assessment (MoCA) score was 25/30, missing three points for delayed recall of five memory words, one point for numbering on a clock face, and two points for abstraction. On the same day, 1.5-Tesla (1.5T) magnetic resonance imaging (MRI) of the brain revealed FLAIR hyperintensity and restricted diffusion throughout the bilateral hippocampi, without involvement of other brain areas (Figure 1A-C). Ten days after symptom onset, the patient had a normal electroencephalogram.
Figure 1.
Twenty-one days after symptom onset, the patient and his family stated that his memory was still improving but had not yet returned to baseline. To clarify the range and severity of cognitive deficits, beyond what may be achieved with the MoCA, we performed a comprehensive neuropsychological evaluation and found significant, isolated deficits of verbal delayed episodic memory function (Table 2). On the same day, dedicated ultra-high field 7-Tesla (7T) MRI of the hippocampus (Kerchner et al., 2012), available as part of a research protocol, revealed no abnormal T2-weighted signal or other abnormality (Figure 1D), consistent with interval improvement from the prior study. Shortly thereafter, he returned to full-time work.
Table 2.
Neuropsychological Testing
| Test Description | Day 21 | Day 56 |
|---|---|---|
| General Intelligence | ||
| WASI Full-4 IQ* | 66 | |
| WASI Verbal IQ* | 50 | |
| WASI Performance IQ* | 73 | |
| Working Memory and Learning | ||
| WMS-III Digit Span* | 99 | 99 |
| WMS-III Spatial Span* | 98 | 98 |
| HVLT-R Immediate Recall* | 55 | 63 |
| WMS-III Logical Memory I* | 16 | 37 |
| BVMT-R Immediate Recall* | 18 | 50 |
| Delayed Episodic Memory | ||
| HVLT-R Delayed Recall* | < 1*** | 63 |
| WMS-III Logical Memory II* | < 1*** | 16 |
| BVMT-R Delayed Recall* | 84 | 84 |
| Executive Function | ||
| Trail Making Test | ||
| Part A** | 24 | 86 |
| Part B** | 50 | 98 |
| Victoria Stroop Test, Interference* | 75 | 98 |
| Letter Fluency** | 39 | 19 |
Test scores expressed as age-adjusted percentiles
Test scores expressed as age- and education-adjusted percentiles
Impairment, defined as ≥2 standard deviations below average
Abbreviations: WASI, Wechsler Abbreviated Scale of Intelligence; HVLT-R, Hopkins Verbal Learning Test–Revised; WMS-III, Wechsler Memory Scale, 3rd Edition; BVMT-R, Brief Visuospatial Memory Test–Revised
Fifty-six days after symptom onset, the patient had his final clinical evaluation. His episodic memory dysfunction had resolved (Table 2). There were interval improvements in other cognitive domains, although he was not deficient in those domains at baseline, suggesting that practice effects may have played a role in these improvements. He was discharged from clinic with a final diagnosis of influenza-associated prolonged global amnesia
Discussion
Our patient developed an amnestic syndrome in the acute phase of influenza A infection. Episodic memory failure was evident at initial presentation and on subsequent neuropsychological tests(Table 2). Imaging revealed evidence of cytotoxic edema diffusely affecting only the bilateral hippocampi. Three weeks after symptom onset high resolution 7T MRI of the hippocampus revealed normal hippocampal morphology without evidence of residual injury (Fig. 1); although the follow-up imaging was done on a different scanner than the initial evaluation, 7T MRI would be expected to be even more sensitive than 1.5T at elucidating subtle T2 signal abnormalities in the hippocampus. Several weeks later, the psychometric abnormality had resolved (Table 2). Together, both neuropsychological and imaging data point to a transient syndrome of hippocampal dysfunction.
Cerebral dysfunction associated with influenza infection has primarily been described in relation to the major influenza pandemics of the past two centuries. Post-infectious complications are most commonly reported. Among the most notable, Encephalitis Lethargica (EL) is a clinical syndrome marked by symptoms of high fever, oculomotor dysfunction, confusion, somnolence or insomnia, hyperkinetic movement disorders, and in many cases, death due to respiratory failure. Postencephalitic Parkinsonism (PEP) and oculogyric crisis have been described in the subacute phase of EL. Influenza is suspected to play a role in the pathophysiology of at least some cases of EL and PEP, although the association remains controversial (Dickman, 2001). Other post-infectious complications include encephalitis and encephalomyelitis, as well as a wide range of transient symptoms not associated with an inflammatory profile on CSF or imaging (Henry, 2010). Neurological symptoms during acute infection are rare. Influenza-associated acute encephalopathy (IAE) is a recently recognized syndrome that was initially reported among Japanese and Taiwanese children, but has subsequently been described in children and adults from diverse ethnic backgrounds. Symptoms include high fever, lethargy, aphasia, convulsions, and headache. Unlike Reye’s syndrome, it is not associated with use of salicylates or hepatic steatosis. The initial neurologic symptoms of IAE typically coincide with respiratory symptoms, and complete recovery usually occurs over weeks to months. CSF pleocytosis is unusual, although influenza RNA may be detected in the CSF by PCR (Wang, Li, & Li, 2010; Davis, 2010; Fujimoto et al., 1998). Imaging is typically unremarkable, although diffuse hemispheric edema with restricted diffusion, thalamic necrosis, brainstem and cerebellar lesions, and cerebral vasculopathy have been described in acute necrotizing encephalopathy (ANE), a severe variant with a high degree of mortality (Akins et al., 2010).
In our patient, imaging and neuropsychological testing point to predominant hippocampal involvement. Although it is difficult to drawn definitive conclusions about the mechanism of our patient’s hippocampal injury with the available data, we suspect that he developed an aberrant parainfectious response to influenza A similar to one proposed mechanism of IAE/ANE(Wang et al., 2010). Consistent with this suspicion, a recent study in miceinfected by intranasal inoculation with a non-neurotropic influenza strain displayed specific memory deficits, elevation of hippocampal cytokines produced by activated microglia, and changes in hippocampal neuronal morphology without detection of viral RNA in the hippocampi during the acute phase of infection (Jurgens, Amancherla, & Johnson, 2012).
For several reasons, we think that our patient’s presentation is unlikely to be due to direct viral invasion by influenza. Rapid resolution of T2-weighted MRI abnormalities is inconsistent with the imaging pattern seen in most neuroinvasive viral infections (Osborn, 1994). IHighly pathogenic strains of influenza, such as H5N1 (avian flu), may cross the blood-brain barrier during infection and cause neurologic injury; however, the pattern generally follows a typical progression, with widespread inflammation and injury of other CNS structures prior to hippocampal involvement (Gao et al., 2010; Jang et al., 2009). Humans rarely contract avian flu in North America (Centers for Disease Control and Prevention [CDC], 2012). Finally, the strains most commonly isolated during the US 2011-2012 influenza season (H1 and H3) are not known to cross the blood-brain barrier (Davis, 2010; CDC, 2012). Other less likely etiologies for this presentation include co-infection with or reactivation of a latent virus with hippocampal tropism (such as HSV-1, HHV-6, or HHV-7) (Ball, 2003; Sugaya et al., 2002), prolonged postictal amnesia (Maheu, Adam, Hazemann, Baulac, & Samson, 2004), persistent seizure activity limited to the mesial temporal lobes (Lee et al., 1992), or a separate and undetected toxic or metabolic disorder (Forster et al., 2012).
Our report of a patient with rapid-onset prolonged amnestic syndrome and transient hippocampal imaging abnormalities expands the clinical spectrum of neurologic manifestations during the acute phase of influenza infection. Influenza should be considered when evaluating a patient with a focal or global cognitive deficit associated with a febrile illness.
Acknowledgements
This research was supported by funds from the National Institute of Health NS065116 (AAK).
References
- 1.Henry J, Smeyne RJ, Jang H, Miller B, Okun MS. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism & Related Disorders. 2010;16:566–571. doi: 10.1016/j.parkreldis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerchner GA, Deutsch GK, Zeineh M, Dougherty RF, Saranathan M, Rutt BK. Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer’s disease. Neuroimage. 2012;63:194–202. doi: 10.1016/j.neuroimage.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickman MS. Von Economo encephalitis. Arcchives of Neurology. 2001;58:1696–1698. doi: 10.1001/archneur.58.10.1696. [DOI] [PubMed] [Google Scholar]
- 4.Wang GF, Li W, Li K. Acute encephalopathy and encephalitis caused by influenza virus infection. Current Opinion in Neurology. 2010;23:305–311. doi: 10.1097/wco.0b013e328338f6c9. [DOI] [PubMed] [Google Scholar]
- 5.Davis L. Neurologic and Muscular Complications of the 2009 Influenza A (H1N1) Pandemic. Current Neurology and Neuroscience Reports. 2010;10:476–483. doi: 10.1007/s11910-010-0135-1. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto S, Kobayashi M, Uemura O, Mitsuji I, Tsunesaburo A, Toshiyuki K, Yoshiro W. PCR on cerebrospinal fluid to show influenza-associated acute encephalopathy or encephalitis. Lancet. 1998;352:873–875. doi: 10.1016/S0140-6736(98)12449-2. [DOI] [PubMed] [Google Scholar]
- 7.Akins PT, Belko J, Uyeki TM, Axelrod Y, Lee KK, Silverthorn J. H1N1 encephalitis with malignant edema and review of neurologic complications from inluenza. Neurocritical Care. 2010;13:396–406. doi: 10.1007/s12028-010-9436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. Journal of Neuroscience. 2012;32:2958–2968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osborn A. Diagnostic Neuroradiology. Mosby; Maryland Heights, MO: 1994. [Google Scholar]
- 10.Gao R, Dong L, Dong J, Wen L, Zhang Y, Yu H, Shu Y. A systematic molecular pathology study of a laboratory confirmed H5N1 human case. PloS ONE. 2010;5:e13315. doi: 10.1371/journal.pone.0013315. doi:10.1371/journal.pone.0013315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang H, Boltz D, Sturm-Ramirez K, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster C, Smeyne RJ. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14063–14068. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Avian Influenza Current Situation. 2012 Jun 21; Retrieved from: http://www.cdc.gov/flu/avianflu/avian-flu-summary.htm.
- 13.Centers for Disease Control and Prevention 2011-2012 Influenza Season Week 37 ending September 15, 2012. 2012 Sep 21; Retrieved from: http://www.cdc.gov/flu/weekly/weeklyarchives2011-2012/weekly37.htm.
- 14.Ball MJ. Unexplained sudden amnesia, postencephalitic parkinson disease, subacute sclerosing panencephalitis, and alzheimer disease: Does viral synergy produce neurofibrillary tangles? Archives of Neurology. 2003;60:641–642. doi: 10.1001/archneur.60.4.641. [DOI] [PubMed] [Google Scholar]
- 15.Sugaya N, Yoshikawa T, Miura M, Ishizuka T, Kawakami C, Asano Y. Influenza encephalopathy associated with infection with human herpesvirus 6 and/or human herpesvirus 7. Clinical Infectious Diseases. 2002;34:461–466. doi: 10.1086/338468. [DOI] [PubMed] [Google Scholar]
- 16.Maheu G, Adam C, Hazemann P, Baulac M, Samson S. A case of postictal transient anterograde and retrograde amnesia. Epilepsia. 2004;45:1459–1460. doi: 10.1111/j.0013-9580.2004.11604.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee BI, Lee BC, Hwang YM, Sohn YH, Jung JW, Park SC, Han MH. Prolonged ictal amnesia with transient focal abnormalities on magnetic resonance imaging. Epilepsia. 1992;33:1042–1046. doi: 10.1111/j.1528-1157.1992.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 18.Forster A, Griebe M, Gass A, Kern R, Hennerici MG, Szabo K. Diffusion-weighted imaging for the differential diagnosis of disorders affecting the hippocampus. Cerebrovasc Dis. 2012;33:104–115. doi: 10.1159/000332036. [DOI] [PubMed] [Google Scholar]