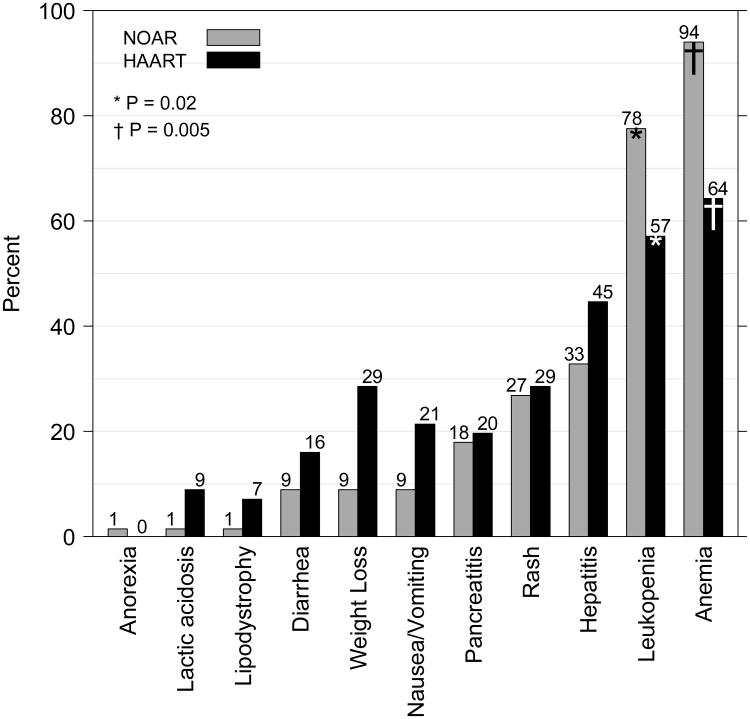
Figure 2.
Percentages of nucleoside reverse transcriptase inhibitor only antiretroviral regimen (NOAR)-treated and highly active antiretroviral therapy(HAART)-treated participants with adverse events reported at any time during the treatment period. LEGACY cohort, 2001-2006.
Note: Only P-values < 0.05 are shown. P-values noted above represent comparisons between the NOAR and HAART columns for leukopenia(*) and anemia(†).
P-values for other adverse events are ≥ 0.05.