Abstract
The increasing prevalence of metabolic syndrome (MS) poses a serious public health problem worldwide. Effective prevention and intervention require improved understanding of the factors that contribute to MS. We analyzed data on a large twin cohort to estimate genetic and environmental contributions to MS and to major MS components and their inter-correlations: waist circumference, systolic and diastolic blood pressure, fasting plasma glucose, triglycerides, and high density lipoprotein cholesterol. We applied structural equation modeling to determine genetic and environmental structure of MS and its major components, using 1,617 adult female twin pairs recruited from rural China. The heritability estimate for MS was 0.42 (95% CI: 0.00–0.83) in this sample with low MS prevalence (4.4%). For MS components, heritability estimates were statistically significant and ranged from 0.13 to 0.64 highest for WC, followed by TG, SBP, DBP, HDL-C, and FPG. HDL-C was mainly influenced by common environmental factors (0.62, 95%CI: 0.58–0.62), while the other five MS components were largely influenced by unique environmental factors (0.32–0.44). Bivariate Cholesky decomposition analysis indicated that the clinical clustering of MS components may be explained by shared genetic and/or environmental factors. Our study underscores the importance of examining MS components as inter-correlated traits, and to carefully consider environmental and genetic factors in studying MS etiology.
Keywords: metabolic syndrome, twin study, heritability, Chinese
INTRODUCTION
The metabolic syndrome (MS, also called insulin resistance syndrome) is defined as a cluster of metabolic abnormalities including central obesity, hypertension, hyperglycemia, and dyslipidemia(1). As the prevalence of MS has increased rapidly in the past few decades, it has become one of the major public-health challenges worldwide(2). MS has been associated with increased risk of cardiovascular disease, stroke and type 2 diabetes(3–5). However, the etiology of MS remains to be elucidated.
Rapid rises in obesity and associated MS suggest that social, physical and environmental factors and lifestyle are important contributors. Indeed, epidemiological studies showed that older age(6–8), western-style diet(9), sedentary lifestyle(10), and physical inactivity(10, 11) increase the risk of MS. On the other hand, considerable individual variability in MS phenotypic components is observed among the subjects living in the same environment. Family and twin studies indicate that genetic determinants also play an important role in the development of MS(12–14).
The twin study design has a long-standing history of usefulness in studying the relative roles of genetic and environmental factors in the development of diseases and phenotypic traits(15). Heritability is an estimated measure of the genetic contribution to total phenotypic variance. To date, most twin studies addressing the heritability of MS components(16–18) were carried out in white populations, with modest sample sizes (e.g. 289–625 twin pairs)(16–18). The reported heritability of MS components varied: waist circumference (0.40–0.63), fasting plasma glucose (0.07–0.28), TG (0.20–0.47), HDL-C (0.43–0.63), systolic blood pressure (0.28–1.00) and diastolic blood pressure (0.04–0.62)(16–22).
To our knowledge, this study is the first and the largest twin study of MS and its phenotypic components in rural Chinese women. Chinese population comprises a fifth of the world’s population, with approximately 80% Chinese reside in rural areas. China is undergoing rapid economic and nutritional changes, which are accompanied by rapidly rising rates of obesity, type 2 diabetes and MS. Yet to date, no study on genetic and environmental contributions to MS and its phenotypic components has been conducted in Chinese rural areas, where the majority of Chinese population lives. For these reasons, studies in the Chinese population may provide unique insights into how genetic and environmental factors influence the MS components during rapid economic and nutritional changes, and how comparable of the variance component estimates to those in the western populations.
This study also attempts to contribute new information to the MS field. Although MS, by definition, consists of multiple components that tend to cluster in the same individuals, it is not yet known if that clustering is due to environmental and/or genetic factors. A Swedish twin study used cross-twin, cross-trait correlations to demonstrate that MS component phenotypes are, at least in part, caused by common genetic and /or environmental factors(18). However, the extent to which the correlated MS components share the common source of variance was not quantified.
Using a large population-based rural Chinese twin cohort, the central focus of our study was to estimate the extent of genetic and environmental influence on MS and major MS phenotypic components. This study also evaluated to what degree the inter-correlations among the major MS phenotypic components are governed by shared genetic and /or environmental factors.
MATERIALS AND METHODS
Study Sample Population and Procedures
The study sample is part of a community-based twin cohort that was recruited in the Anqing and Luan areas, Anhui Province, China from September 1998 to May 2000. The Anqing area is located on the north bank of the Yangtze River in Anhui Province, China, which has one city, two suburban areas and eight rural counties, with a total population of 6.1 million (90% rural). The Luan area lies north of the Dabie Mountain in Anhui Province. There are one city, two suburban areas, and five rural counties in the area, with a total population of 6.6 million (93% rural). Inclusion criteria for twins in the original study were: (1) ages 6 to 60 years; (2) both twins available; and (3) both twins (or parents/guardians of children) agreed and consented to participate; (4) no history of stroke and cardiovascular, renal, hepatic or malignant diseases; and (5) females were not nursing or pregnant. All study subjects were of Han ethnicity based on self-report.
Eligible twin pairs were invited to a central office to complete a dual energy X-ray absorptiometry scan (DEXA) and physical exam, including anthropometric measures. Data on smoking status, alcohol consumption, occupation and education were collected with a standardized questionnaire, administered by well-trained interviewers. To obtain a more homogeneous study population, this report is limited to adult female twin pairs. A total of 1617 adult female twin pairs aged 20 to 60 years of age completed MS components measurements and zygosity determination, and were included in this analysis. The study protocol was approved by the Institutional Review Boards at Children's Memorial Hospital and the Institute of Biomedicine, Anhui Medical University in Hefei, China.
Anthropometry
Height was measured without shoes to the nearest 0.1 cm on a portable stadiometer. Weight was measured without shoes to the nearest 0.1 kg with the subject standing motionless in the center of a calibrated scale. Waist circumference (WC) was measured as the minimum circumference between the inferior margin of the ribcage and the crest of the ileum. The mean value of three measurements was used.
Blood Pressure
Blood pressure was measured by a nurse according to standard procedure. Briefly, the right arm was used for all blood pressure measurements, with the cubital fossa supported at heart level after the subject had voided, rested, and been seated comfortably for 10 minutes. A standard clinical sphygmomanometer was used, with the bell of the stethoscope placed over the brachial artery pulse, proximal and medial to the cubital fossa and below the bottom edge of the cuff. Systolic blood pressure was defined as Korotkoff phase I (appearance of sound), and diastolic blood pressure was defined as Korotkoff phase V (disappearance of sound). Three measurements were taken for each subject, with at least 30 seconds between readings. The mean of the three values was used in subsequent analyses.
Laboratory Assays
Serum and plasma were separated from blood cells in the field within 30 minutes and kept frozen at −80 °C. Fasting plasma glucose was measured within 2 hours of the blood draw by a modified hexokinase enzymatic method using a XD-811 Semi-automatic Analyzer (Xunda Corporation, Shanghai, China). Serum total cholesterol (TC), triglyceride (TG), and high-density lipoprotein-cholesterol (HDL-C) were also measured on the XD-811 Semi-automatic Analyzer.
Zygosity Ascertainment
Twin zygosity was determined using 10 autosomal polymorphic microsatellite markers, which met the following criteria: Twin zygosity was determined using 10 autosomal polymorphic microsatellite markers, which met the following criteria: a. With higher heterozygosity frequency (≥0.70) based on multiple previous large genetic epidemiology studies of the same population; b. each marker is located in a separate autosome; c. The markers were commercial available (23). 5' Fluorescent Labeled Oligo primers for each marker was ordered from ABI(Foster City, CA). The markers' genotypes were identified by electrophoresis using an ABI 3730 DNA Analyzer. Genotyping quality control included 4 repeat samples, random selection of 15% of the sample for repeat genotyping, and analyses of two commercial standard DNA samples with identified genotypes from the National Institute of General Medical Sciences. Twin-pair MZ scores greater than 0.995 were defined as monozygotic. If any different markers’ genotype was observed between twin-pair, the twin-pair was classified as dizygotic.
Definitions of Metabolic Syndrome
Modified ATP-III criteria for MS have been proposed and used in several Asian population studies(24, 25). This study adopted the modified criteria. Specifically, a female MS case should meet three or more of the following conditions: waist circumference ≥ 80 cm (larger waist circumference); systolic blood pressure (SBP) ≥130 mm Hg or diastolic blood pressure (DBP) ≥85 mm Hg (higher blood pressure); fasting plasma glucose (FPG) ≥ 6.1 mmol/l (raised FPG); triglyceride ≥1.7 mmol/l (raised serum TG); and high density lipoprotein-C < 1.29 mmol/l (low serum HDL-C).
Statistical Analysis
We applied generalized estimating equations (GEE) to compare the differences of MS components between zygosity groups. Due to skewed distribution, loge – transformed TG and HDL-C (i.e. lnTG and LnHDL) were used in the analyses. Age-adjusted Pearson’s partial-correlation analyses were applied to calculate intraclass correlation for continuous outcomes within twin pairs, stratified by zygosity. All analyses were performed using SAS software, version 9.0 (SAS Institute, Cary, North Carolina).
The classical twin study compares phenotypic resemblances of MZ and DZ twins. In the simplest term, the extent to which monozygotic (MZ) twin pairs are more similar than dizygotic (DZ) twin pairs for a trait reflects the genetic contribution to the population variance in that trait because MZ twins share all their genes, whereas DZ twins share, on average, only half of their segregating genes. Structural equation modeling in Mx(26) was used to estimate genetic and environmental influences on MS and its components in this twin study. In particular, we fitted a model that allowed for additive genetic (a2), common environmental (c2), and unique environmental (e2) components. 95% confidence intervals (CIs) were calculated for the parameter estimates; when these included 0, the parameters were interpreted as not statistically significant. We did not perform model comparisons by restricting a, c, or e as zero, since a complex trait was likely to be influenced by all these factors and a statistically best model may not count this biological basis. Finally, with Mx, we fitted the bivariate Cholesky decomposition models to calculate genetic and environmental correlations among MS components. The phenotypic correlations between MS component pairs can be expressed as:
where rG, rC and rE are the additive genetic correlation, common environmental correlation and unique environmental correlation between each MS component pair, respectively; h12 and h22 are additive genetic components; c12 and c22 are common environmental components; and e12 and e22 are unique environmental components for each tested pair. The genetic (CGCP), common (CCCP) and unique (CUCP) environmental contributions to the phenotypic correlations are and , respectively.
RESULTS
A total of 1,617 female twin pairs aged 20 to 60 years (1116 MZ and 501 DZ) were included in this study. The demographic and clinical characteristics of these twins are summarized in Table 1. The mean age was 32.3 years. Compared with western populations, this population was relatively lean, with a mean BMI of 21.9 kg/m2. The majority (76.8%) of the twins were farmers and most of them reported no current smoking or alcohol consumption. There was no significant difference between MZ and DZ twins on any of the variables shown.
Table 1.
Demographic characteristics and the prevalence of MS and Its Components in Chinese female twins, aged from 20 to 60 years.
| Variables¶ | MZ (1116 pairs) |
DZ (501 pairs) |
Total (1,617 pairs) |
|---|---|---|---|
| Mean ± SD | |||
| Age, yr | 32.4±7.8 | 32.1±8.1 | 32.3±7.9 |
| Body Mass Index (BMI), kg/m2 | 22.0±2.8 | 21.8±2.7 | 21.9±2.8 |
| Waist Circumference (WC), cm | 71.8±7.7 | 71.6±7.8 | 71.7±7.7 |
| Systolic Blood Pressure (SBP), mmHg | 111.6±12.6 | 112.6±13.4 | 111.9±12.8 |
| Diastolic Blood Pressure(DBP), mmHg | 66.0±8.5 | 66.5±9.2 | 66.2±8.7 |
| Fasting Blood Glucose (FPG), mM | 4.55±0.87 | 4.58±0.85 | 4.56±0.86 |
| High Density Lipid (HDL), mM | 1.55±0.70 | 1.49±0.58 | 1.53±0.67 |
| Triglyceride (TG), mM | 0.89±0.62 | 0.85±0.59 | 0.88±0.61 |
| N (%) | |||
| Farmer | 1706(77.5) | 774(76.5) | 2480(76.8) |
| <High school | 2103(94.2) | 926(92.4) | 3029(93.7) |
| Current Smoking | 31(1.4) | 10(1.0) | 41(1.3) |
| Current Alcohol Drinking | 57(2.6) | 22(2.2) | 79(2.4) |
| Larger Waist Circumference | 354(15.9) | 141(14.1) | 495(15.3) |
| Higher BP | 162(7.3) | 86(8.6) | 248(7.7) |
| Raised FPG | 119(5.3) | 49(4.9) | 168(5.2) |
| Raised TG | 179(8.0) | 65(6.5) | 244(7.5) |
| Lower HDL-C | 780(35.0) | 324(32.3) | 1104(34.1) |
| >=1 MS Components | 1117(50.0) | 483(48.2) | 1600(50.5) |
| Metabolic Syndrome (MS) | 101(4.5) | 42(4.2) | 143(4.4) |
Continuous variables are presented as mean ± standard deviation. Binary variables are summarized by N and percentage. Generalized estimating equations (GEE) were applied to compare the differences between MZ and DZ for all variables. TG and HDL-C were transformed by natural logarithm (i.e. lnTG and LnHDL) for all statistical analyses. Larger waist circumference denotes waist circumference≥80 cm; higher BP denotes SBP≥130 or DBP≥85 mmHg; raised FPG denotes FPG (6.1mM; raised TG denotes triglyceride (1.70mM/l; lower HDL-C denotes HDL<1.29mM/l. MS are defined as the subjects who meet the modified ATP-III Criteria. No significant difference was found for all variables between MZ and DZ subjects.
MS was present in 4.4% of total study subjects. However, 50.5% met one or more MS phenotypic components. The prevalence of various MS components morbidities were: 34.1% for low HDL-C, 15.3% for large waist circumference, 7.7% for high blood pressure, 7.5% for elevated TG, and 5.2% for elevated FPG. There were no significant differences between MZ and DZ twins. Given the low prevalence for some of the dichotomized MS components, our analyses and presentations focused on continuous MS phenotypes.
Table 2 shows the variance component estimates for each MS phenotypic component. The intraclass correlation coefficients of the MS components are higher among MZ than among DZ pairs, suggesting that genetic factors may be important contributors to these traits. Heritability estimates ranged from 0.13 to 0.64, highest for WC 0.64 (95%CI: 0.49~0.71), followed by TG 0.50 (95%CI: 0.34~0.62), SBP 0.42 (95%CI: 0.29~0.56), DBP 0.40 (95%CI: 0.25~0.55), HDL-C 0.22 (95%CI: 0.15~0.30), and FPG 0.22 (95%CI: 0.08~0.37). The heritability estimates of metabolic syndrome itself is 0.42 (95%CI: 0.00~0.83), which is not statistically significant. Note that, common and unique environmental factors also contribute to the phenotypic variance of MS components: common environment effects account for 62% (95%CI: 0.58 – 0.62) of HDL-C variance; unique environment effects account for 30–40% of the variance of the other 5 MS components.
Table 2.
Heritability Estimates for MS Components in 1617 Female Twin Pairs.
| Variables | Intraclass correlations* | Parameter Estimates¶ | |||
|---|---|---|---|---|---|
| MZ | DZ | h2 (95%CI) | c2 (95%CI) | e2 (95%CI) | |
| WC, cm | 0.68 | 0.37 | 0.64(0.49~0.71) | 0.05(0.00~0.18) | 0.32(0.29~0.35) |
| SBP, mmHg | 0.64 | 0.43 | 0.42(0.29~0.56) | 0.25(0.12~0.37) | 0.33(0.30~0.36) |
| DBP, mmHg | 0.55 | 0.36 | 0.40(0.25~0.55) | 0.19(0.05~0.32) | 0.41(0.38~0.45) |
| FPG, mM | 0.56 | 0.45 | 0.22(0.08~0.37) | 0.35(0.20~0.47) | 0.44(0.40~0.48) |
| TG, mM | 0.60 | 0.32 | 0.50(0.34~0.62) | 0.09(0.00~0.25) | 0.41(0.38~0.45) |
| HDL-C, mM | 0.85 | 0.68 | 0.22(0.15~0.30) | 0.62(0.58~0.62) | 0.16(0.16~0.18) |
Age-adjusted pearson partial correlation analysis was used to calculate the intraclass correlations within twin pairs, TG and HDL-C were transformed by natural logarithm. All correlations were statistical significantly (p<0.001).
ACE models were used for all the h2 parameter estimates
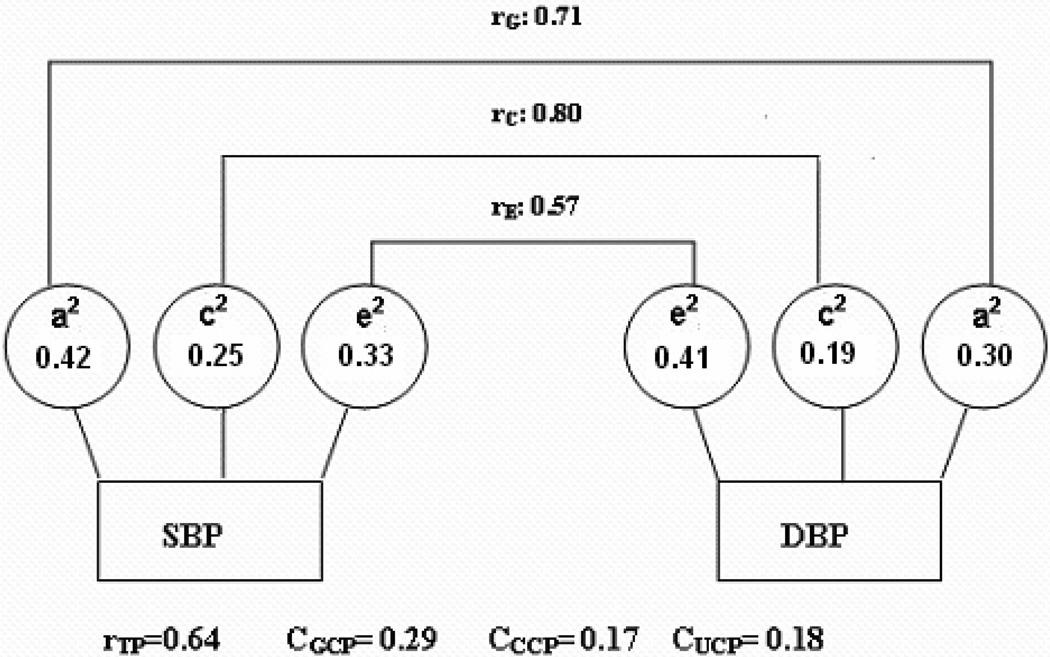
Table 3 shows the quantitative contribution of genetic and environmental factors to the phenotypic correlation of MS component pairs. Figure 1 uses SBP and DBP as an example to illustrate how to understand the estimates. The genetic, common and unique environmental components all contribute to the total variance of SBP, each with moderate or weak effects (h2=0.42, c2=0.25, and e2=0.33 in Table 2). Similar estimates were observed for the total variance of DBP. Bivariate Cholesky decomposition revealed high genetic (rG=0.71) and environmental correlations (rC=0.80; rE=0.57) between SBP and DBP, indicating that these two measures share some genetic and environmental factors. However, the quantitative contributions of each component to the covariance between SBP and DBP are only 0.29, 0.17, and 0.18, respectively, based on the effects estimates of each trait and the corresponding correlations between two traits (See Equation in Methods and Table 3 footnotes). In other words, 45% (=0.29/0.64) of the total phenotypic correlation between SBP and DBP is due to genetic factors, 27% (=0.17/0.64) is due to common environmental factors, and 28% (=0.18/0.64) is due to unique environmental factors. The phenotypic correlations between SBP and WC, DBP and WC are quite small (0.17 and 0.23). Nevertheless, common environmental factors explain more than 50% of these small correlations. More interestingly, most of phenotypic correlation between WC and TG is explained by genetic effects. Here, we present detailed interpretation of how a variance component contributes to covariance of the phenotype pairs only for pairs with higher correlation coefficients (rTP>0.15). In the phenotypic pairs with lower phenotypic correlation, most of the variance components correlations were not statistically significant (i.e., 95% CIs include 0). Unique environmental correlations were quite small, although in general were statistically significant.
Table 3.
Genetic and environmental contributions to phenotypic correlations among MS components in 1617 Chinese Female Twin Pairs.
| Variables | Variance components correlations among MS phenotypic traits |
Genetic and environmental contributions to phenotypic correlations |
|||||
|---|---|---|---|---|---|---|---|
| rG | rC | rE | rTP | CGCP | CCCP | CUCP | |
| SBP—DBP | 0.71(0.56~0.83) | 0.80(−1.00~1.00) | 0.57(0.53~0.62) | 0.64 | 0.29 | 0.17 | 0.18 |
| SBP—WC | 0.00(−0.18~0.17) | 1.00(0.57~1.00) | 0.19(0.13~0.24) | 0.17 | 0.00 | 0.11 | 0.06 |
| SBP—TG | 0.22 (−0.01~0.44) | −0.34(−1.00~1.00) | 0.07(0.01~0.13) | 0.08 | 0.10 | −0.05 | 0.03 |
| SBP—HDL | 0.06(−0.17~0.29) | −0.20(−0.44~0.04) | 0.06(−0.05~0.06) | −0.06 | 0.02 | −0.08 | 0.01 |
| SBP—FPG | 0.05(−0.31~0.38) | 0.27(0.07~1.00) | 0.05(0.01~0.11) | 0.11 | 0.01 | 0.08 | 0.02 |
| DBP—WC | 0.13(−0.06~0.31) | 1.00(−0.69~1.00) | 0.17(0.11~0.22) | 0.23 | 0.07 | 0.10 | 0.06 |
| DBP—TG | 0.19(−0.06~0.42) | −0.18(−1.00~1.00) | 0.12(0.07~0.18) | 0.11 | 0.08 | −0.02 | 0.05 |
| DBP—HDL | −0.16(−0.31~0.09) | −0.08(−0.88~1.00) | 0.07(0.02~0.10) | −0.06 | −0.05 | −0.03 | 0.02 |
| DBP—FPG | 0.08(−0.29~0.44) | 0.27(0.07~1.00) | 0.05(0.01~0.11) | 0.11 | 0.02 | 0.07 | 0.02 |
| WC—TG | 0.40(0.22~0.55) | −1.00(−1.00~1.00) | 0.20(0.14~0.25) | 0.23 | 0.23 | −0.07 | 0.07 |
| WC—HDL | −0.13(−0.30~0.07) | −0.25(−1.00~0.98) | −0.10(−0.15~−0.05) | −0.11 | −0.05 | −0.04 | −0.02 |
| WC—FPG | 0.01(−0.30~0.31) | 0.36(0.36~0.90) | 0.06(0.00~0.17) | 0.07 | 0.00 | 0.05 | 0.02 |
| TG—HDL | −0.24(−0.46~−0.01) | 0.17(−1.00~1.00) | −0.21(−0.27~−0.16) | −0.11 | −0.08 | 0.04 | −0.05 |
| TG—FPG | −0.08(−0.47~0.27) | 0.33(−1.00~1.00) | 0.04(−0.02~0.10) | 0.05 | −0.03 | 0.06 | 0.02 |
| HDL—FPG | 0.08(−0.28~0.47) | −0.19(−0.38~−0.02) | −0.04(−0.10~0.02) | 0.09 | 0.02 | 0.09 | −0.02 |
¡ rG, Genetic correlation among MS components; rC, Common environmental correlation among MS components; rE, unique environmental correlation among MS components.
¡¡ rTP, total phenotypic correlation among MS components; CGCP, Genetic contribution to the phenotypic correlation rTP; CCCP, common environmental contribution to the phenotypic correlation rTP; CUCP, unique environmental contribution to the phenotypic correlation rTP.
¡¡¡ rTP= CGCP+ CSCP+ CUCP, , where h2s, c2s, e2s were given in table 2.
Figure. 1.
Genetic and environmental contributions to the phenotypic correlations of SBP and DBP in 1617 female Chinese twin pairs adjusted with age; rG, rC and rE, genetic, common, and unique environmental correlation; a2, c2, and e2, percentage of total phenotypic variance accounted for by genetic factors, common environmental factors, and unique environmental factors; rTP, total phenotypic correlation; CGCP, Genetic contribution to total phenotypic correlation; CCCP, common environmental contribution to total phenotypic correlation; CUCP, unique environmental contribution to total phenotypic correlation.
Finally, we separately analyzed pre- and post- menopausal twins. The results were consistent with the findings presented above (data not shown).
DISCUSSION
To our knowledge, the present study is among the largest twin studies of MS and its phenotypic components. As such, it should provide the best available power for determining the genetic and environmental influence. So far, most previous twin studies in this field are conducted in Western populations(16–18). The present study is the first and the largest female twin study that addresses environmental and genetic contribution to MS and its components in a Chinese rural population. Approximately 80% of Chinese reside in rural areas, and thus our study contributed to the knowledge on a huge but understudied population in the world. As shown in our previous publication(27), this twin population is similar to the local general population in terms of sociodemographic characteristics, life style, and anthropometric measurements. Our study findings are likely generalizable to local rural female population. However, cautions are needed to generalize the findings from this study to males and urban population in China.
The prevalence of MS is low in this population (4.4%), thus resulting in a non-significant heritability estimate. However, this estimated genetic component of MS (0.42) in our female twin sample was consistent with the findings from a previous study in 179 Hong Kong Chinese families with early-onset type 2 diabetes and those from other ethnic groups (0.24–0.43)(19–21). In addition, similar to the previous reports(16–22), most MS components, i.e., waist circumference, SBP, DBP, and TG, appear to be under considerable additive genetic and unique environmental influences. But for HDL-C and FPG, phenotypic variance seems to be largely explained by common environmental factors besides genetic and unique environmental influences. The findings are generally in agreement with previous studies, which suggest that FPG may be affected largely by non-genetic factors(21) and common environment was a significant contributor only for HDL-C among all lipids variables(28). Of note, twin model assumes that the shared environments are equal for both MZ and DZ twins. When this assumption is violated, the estimate of genetic influence might be inflated because MZ twins usually experience more similar environments than DZ twins. Poulsen et al reported that the differences both in plasma glucose and plasma insulin concentrations during an oral glucose tolerance test were observed between monozygotic and dizygotic twins(29). The findings challenged the equal environment assumption of classical twin study. However, in our study sample, we did not find significant difference in MS phenotypic components between MZ and DZ twins.
Most previous studies analyzed MS components individually, and ignored the fact that these are inter-correlated traits. Clustering of the MS components may be in part due to the shared genetic and/or environmental factors controlling these correlated phenotypes(30–32). Genome-wide linkage studies of MS in Framingham Heart Study have identified several chromosomal regions that were linked with MS and its components, suggesting the existence of common heritable causes(33). In this twin study, we confirmed some genetic and environmental factors underlying correlated MS phenotypic components, consistent with a previous family-based study(22) and a twin study(18). In addition, we quantified the genetic and environmental contributions to the phenotypic correlations.
We observed substantial phenotypic correlation between SBP and DBP, which might be explained by shared genetic and environmental factors controlling both measures of BP. Genetic factors account for most of the total covariance between waist circumference and TG, suggesting that some genetic factors may be shared by these two traits. A candidate for shared genetic factors between waist circumference and TG could be the group of genes involved in the adiponectin pathway. Adiponectin is derived only from adipose tissue and may play an important role in lipid metabolism(34). Epidemiological studies have shown that serum adiponectin concentrations are decreased in patients with obesity(35) and inversely associated with central fat distribution(36) and triglyceride levels(37). Genetic studies have provided evidence that adiponectin gene polymorphisms are associated with body fat distribution(38) and obesity(39). Genetic variants of adiponectin receptor 2 gene are associated with adiponectin levels and triglyceride concentrations in patients with metabolic syndrome(40). Finally, we found substantial environmental contributions to the inter-correlation between waist circumference and SBP and DBP, which might due to some common environmental factors influencing both traits, such as diet, life style and physical activity. Our findings are supported by previous animal studies in which both feeding patterns and the type of diet can influence blood pressure as well as body weight in rats(41, 42).
New insights into the shared environmental and/or genetic factors underlying the correlated MS components may provide better understanding of the etiology of MS and clinically observed clustering of MS components. This can also lead to more precise estimates with lower false positive rates when simultaneously testing the associations between correlated MS components with the risk factors. Our study findings underscore the need to analyze MS phenotypes both individually and simultaneously. Future MS studies are encouraged to apply available statistical methods for this purpose, such as extended generalized estimating equation (EGEE)-based bivariate analytical method(43). (Liu J and Deng HW, Genetic Epidemiology, 2008).
In summary, in this large rural Chinese female twin sample, we demonstrated that both genetic and environmental factors influence MS and its components. The observation that shared genetic and shared environmental factors contribute to the correlated MS phenotypic components underscores the importance of examining MS components as inter-correlated traits. It also emphasizes the importance of carefully considering both environmental and genetic factors in studying the etiology and in developing prevention and treatment strategies for MS. Additional research is needed to identify specific environmental and genetic determinants in this population, which may provide useful information for preventing MS and its associated cardiovascular diseases and, consequently, for reducing global burdens of these chronic diseases.
Acknowledgement
We acknowledge the assistance and cooperation of the faculty and staff of the Anhui Institute of Biomedicine, Anhui Medical University, and thank all study participants for their support.
This study is supported in part by grant R01 HD049059 from the National Institute of Child Health and Human Development; R01 HL0864619 from the National Heart, Lung, and Blood Institute; R01 AG032227 from the National Institute of Aging; and by the Food Allergy Project.
Footnotes
Disclosure Statement: All of the authors listed in this manuscript have nothing to declare.
Reference
- 1.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Wong ND. Metabolic syndrome: cardiovascular risk assessment and management. Am J Cardiovasc Drugs. 2007;7:259–272. doi: 10.2165/00129784-200707040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kurl S, Laukkanen JA, Niskanen L, et al. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke. 2006;37:806–811. doi: 10.1161/01.STR.0000204354.06965.44. [DOI] [PubMed] [Google Scholar]
- 5.Streja D. Metabolic syndrome and other factors associated with increased risk of diabetes. Clin Cornerstone. 2004;6(Suppl 3):S14–S29. doi: 10.1016/s1098-3597(04)80094-2. [DOI] [PubMed] [Google Scholar]
- 6.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: a controlled longitudinal study. Ann Intern Med. 1995;123:673–675. doi: 10.7326/0003-4819-123-9-199511010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Matthews KA, Kuller LH, Sutton-Tyrrell K, et al. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32:1104–1111. doi: 10.1161/01.str.32.5.1104. [DOI] [PubMed] [Google Scholar]
- 9.Baxter AJ, Coyne T, McClintock C. Dietary patterns and metabolic syndrome--a review of epidemiologic evidence. Asia Pac J Clin Nutr. 2006;15:134–142. [PubMed] [Google Scholar]
- 10.Ford ES, Kohl HW, 3rd, Mokdad AH, et al. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes Res. 2005;13:608–614. doi: 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Li C. Physical activity or fitness and the metabolic syndrome. Expert Rev Cardiovasc Ther. 2006;4:897–915. doi: 10.1586/14779072.4.6.897. [DOI] [PubMed] [Google Scholar]
- 12.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 13.Heller DA, de Faire U, Pedersen NL, et al. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993;328:1150–1156. doi: 10.1056/NEJM199304223281603. [DOI] [PubMed] [Google Scholar]
- 14.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 15.Martin N, Boomsma D, Machin G. A twin-pronged attack on complex traits. Nat Genet. 1997;17:387–392. doi: 10.1038/ng1297-387. [DOI] [PubMed] [Google Scholar]
- 16.Benyamin B, Sorensen TI, Schousboe K, et al. Are there common genetic and environmental factors behind the endophenotypes associated with the metabolic syndrome? Diabetologia. 2007;50:1880–1888. doi: 10.1007/s00125-007-0758-1. [DOI] [PubMed] [Google Scholar]
- 17.Poulsen P, Vaag A, Kyvik K, et al. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 2001;44:537–543. doi: 10.1007/s001250051659. [DOI] [PubMed] [Google Scholar]
- 18.Hong Y, Pedersen NL, Brismar K, et al. Genetic and environmental architecture of the features of the insulin-resistance syndrome. Am J Hum Genet. 1997;60:143–152. [PMC free article] [PubMed] [Google Scholar]
- 29.Lin HF, Boden-Albala B, Juo SH, et al. Heritabilities of the metabolic syndrome and its components in the Northern Manhattan Family Study. Diabetologia. 2005;48:2006–2012. doi: 10.1007/s00125-005-1892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayoumi RA, Al-Yahyaee SA, Albarwani SA, et al. Heritability of determinants of the metabolic syndrome among healthy Arabs of the Oman family study. Obesity (Silver Spring) 2007;15:551–556. doi: 10.1038/oby.2007.555. [DOI] [PubMed] [Google Scholar]
- 21.Li JK, Ng MC, So WY, Chiu CK, et al. Phenotypic and genetic clustering of diabetes and metabolic syndrome in Chinese families with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2006;22:46–52. doi: 10.1002/dmrr.577. [DOI] [PubMed] [Google Scholar]
- 22.Freeman MS, Mansfield MW, Barrett JH, et al. Heritability of features of the insulin resistance syndrome in a community-based study of healthy families. Diabet Med. 2002;19:994–999. doi: 10.1046/j.1464-5491.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Necheles J, Ouyang F, et al. Monozygotic co-twin analyses of body composition measurements and serum lipids. Prev Med. 2007 doi: 10.1016/j.ypmed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Chuang SY, Chou P, Hsu PF, et al. Presence and progression of abdominal obesity are predictors of future high blood pressure and hypertension. Am J Hypertens. 2006;19:788–795. doi: 10.1016/j.amjhyper.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Hong X, Li Z, Zhang W, et al. Prevalence of metabolic syndrome and its relation to body composition in a Chinese rural population. Obesity (Silver Spring) 2006;14:2089–2098. doi: 10.1038/oby.2006.244. [DOI] [PubMed] [Google Scholar]
- 26.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, the Netherland: Kluwer Academic; 1992. [Google Scholar]
- 27.Yu Y, Kumar R, Venners S, et al. Age and gender specific lung function predictive equations provide similar predictions for both a twin population and a general population from age 6 through adolescence. Pediatr Pulmonol. 2007 Jul;42(7):631–639. doi: 10.1002/ppul.20631. [DOI] [PubMed] [Google Scholar]
- 28.Goode ELCS, Christian JC, Jarvik GP, et al. Heritability of longitudinal measures of body mass index and lipid and lipoprotein levels in aging twins. Res Hum Genet. 2007;10:703–711. doi: 10.1375/twin.10.5.703. [DOI] [PubMed] [Google Scholar]
- 29.Poulsen P, Vaag A, Beck-Nielsen H. Does zygosity influence the metabolic profile of twins? A population based cross section study. BMJ. 1999;319:151–154. doi: 10.1136/bmj.319.7203.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrannini E, Natali A. Essential hypertension, metabolic disorders, and insulin resistance. Am Heart J. 1991;121:1274–1282. doi: 10.1016/0002-8703(91)90433-i. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt MI, Watson RL, Duncan BB, et al. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Atherosclerosis Risk in Communities Study Investigators. Metabolism. 1996;45:699–706. doi: 10.1016/s0026-0495(96)90134-1. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt MI, Duncan BB, Watson RL, et al. A metabolic syndrome in whites and African-Americans. The Atherosclerosis Risk in Communities baseline study. Diabetes care. 1996;19:414–418. doi: 10.2337/diacare.19.5.414. [DOI] [PubMed] [Google Scholar]
- 33.Goldin LR, Camp NJ, Keen KJ, et al. Analysis of metabolic syndrome phenotypes in Framingham Heart Study families from Genetic Analysis Workshop 13. Genet Epidemiol. 2003;25(Suppl 1):S78–S89. doi: 10.1002/gepi.10288. [DOI] [PubMed] [Google Scholar]
- 34.Havel P. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 35.Arita YKS, Ouchi N, Takahashi M, et al. Paradoxical decrease of an adipose-specific protein, adiponectin,in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 36.Gavrila ACJ, Yiannakouris N, Kontogianni M, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 37.Farvid MSNT, Chan DC, Barrett PH, et al. Association of adiponectin and resistin with adipose tissue compartments, insulin resistance and dyslipidaemia. Diabetes Obes Metab. 2005;7:406–413. doi: 10.1111/j.1463-1326.2004.00410.x. [DOI] [PubMed] [Google Scholar]
- 38.Tankó LBSA, Lecoeur C, Larsen PJ, et al. ACDC/adiponectin and PPAR-gamma gene polymorphisms: implications for features of obesity. Obes Res. 2005;13:2113–2121. doi: 10.1038/oby.2005.262. [DOI] [PubMed] [Google Scholar]
- 39.Menzaghi CET, Di Paola R. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51:2306–2312. doi: 10.2337/diabetes.51.7.2306. [DOI] [PubMed] [Google Scholar]
- 40.Broedl UCLM, Fleischer-Brielmaier E, Tietz AB, et al. Genetic variants of adiponectin receptor 2 are associated with increased adiponectin levels and decreased triglyceride/VLDL levels in patients with metabolic syndrome. Cardiovasc Diabetol. 2006;5:11. doi: 10.1186/1475-2840-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers MM. Enduring effects of infant feeding experiences on adult blood pressure. Psychosom Med. 1996;58:612–621. doi: 10.1097/00006842-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Plagemann A, Harder T, Rake A, et al. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999;836:146–155. doi: 10.1016/s0006-8993(99)01662-5. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Pei Y, Papasian CJ, et al. Bivariate association analyses for the mixture of continuous and binary traits with the use of extended generalized estimating quations. Genet Epidemiol. 2008 doi: 10.1002/gepi.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]