FIGURE 8.
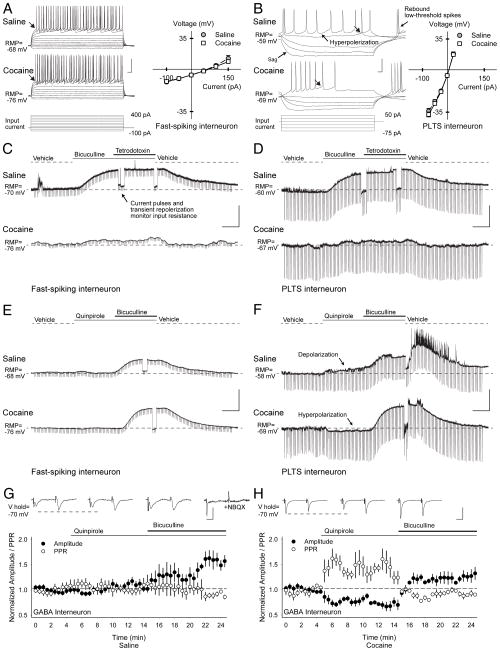
Abnormal GABA interneuron function. (A) Current clamp recordings show characteristic responses of FS and (B) PLTS interneurons from Lhx6-GFP transgenic mice to hyperpolarizing and depolarizing current injections (below). Both FS and PLTS interneurons from cocaine cells had lower resting membrane potentials (RMP) than saline cells. Current-voltage plots (right) show similar responses in saline and cocaine-exposed cells after subtraction of their resting membrane potentials (responses were measured at arrows). FS cells were silent at rest, and displayed a high firing rate with little adaptation following depolarizing current injection. Spikes were short and followed by a large after-hyperpolarization. PLTS interneurons exhibited a marked time-dependent sag in response to hyperpolarizing current injections and a rebound persistent low-threshold spike and/ or a plateau potential persisted after termination of hyperpolarizing current. During current injections, both FS and PLTS interneurons displayed a variable pattern of spike bursts (1–48 action potentials) interspersed by membrane oscillations. Compared to FS interneurons, PLTS interneurons exhibited a much higher input resistance, a lower resting membrane potential (RMP), and a much lower input current was required to produce action potentials (Supplementary Tables S3–S5), with values similar to those reported previously.14,49 (C) Representative current-clamp recordings in FS and (D) PLTS interneurons from saline-(above) and cocaine-exposed mice (below) demonstrate typical responses to the GABAAR antagonist bicuculline before and after bath application of the sodium channel blocker tetrodotoxin. In FS and PLTS interneurons, bicuculline depolarized saline-exposed cells (the RMP became more positive) to a much greater degree than cocaine-exposed cells. GABA likely produced tonic inhibition at GABAA autoreceptors14 since the change in membrane potential by bicuculline in saline-exposed mice (FS, 37±2%, p<0.001; PLTS, 23±7%, p=0.03) and cocaine-exposed mice (FS, 3±1%, p=0.09; PLTS, 4±1%, p=0.003, paired t-test) persisted when synaptic transmission was blocked by tetrodotoxin. Note that the cellular input resistance was monitored by (250 ms, 100 pA) current pulses applied every 10 sec. Changes in input resistance during depolarization were measured after transiently repolarizing the cell to resting membrane potential levels. Interestingly, bicuculline reduced the input resistance (Supplementary Fig S5), suggesting recruitment of additional ion channels with depolarization that are critical for sustained high-frequency firing.39 (E) Representative current-clamp recordings in FS and (F) PLTS interneurons from saline-(above) and cocaine-exposed mice (below) demonstrate typical responses to the D2 receptor agonist quinpirole before and after bicuculline. Quinpirole had no effect in FS interneurons, but slightly depolarized saline-exposed PLTS cells, while hyperpolarizing cocaine-exposed PLTS cells. For all interneurons, the membrane potential became more positive and the cell depolarized when bicuculline was added to quinpirole. A summary of membrane potentials and input resistance for FS and PLTS interneurons under all conditions tested can be found in Supplementary Fig S5. (G) Excitatory inputs onto GFP+ fluorescent interneurons from Lhx6-GFP transgenic mice were activated with paired-pulses using cortical bipolar stimulating electrodes. The PPR was similar in cells from saline (1.35±0.11; n=11) and cocaine-exposed mice (1.13±0.09; n=8; p=0.2, t-test). In saline-exposed mice, representative traces (above) and graph show that quinpirole did not change the amplitude of the first eEPSC (−32±3 pA in vehicle vs. −35±6 pA following quinpirole) or the PPR (1.3±0.1 in vehicle vs. 1.3±0.1 in quinpirole). When quinpirole was combined with bicuculline, the eEPSC amplitude increased (−45±6 pA), but the PPR remained unchanged (1.3±0.3). The AMPA-receptor antagonist NBQX (10 μM) abolished the eEPSC. (H) In GFP+ interneurons from cocaine-exposed mice, quinpirole reduced the amplitude of the first eEPSC (−62±5 pA in vehicle vs. −49±9 pA following quinpirole) and the PPR increased (1.1±0.1 in vehicle vs. 1.4±0.2 in quinpirole). Paired-pulse depression was observed in the presence of bicuculline, which prevented the change in PPR (1.1±0.2) and the eEPSC amplitude increased (−95±13 pA). Bars: A and B, 30 mV, 20 ms; C–F, 30 mV, 2 min; G and H, 50 pA, 12.5 ms.