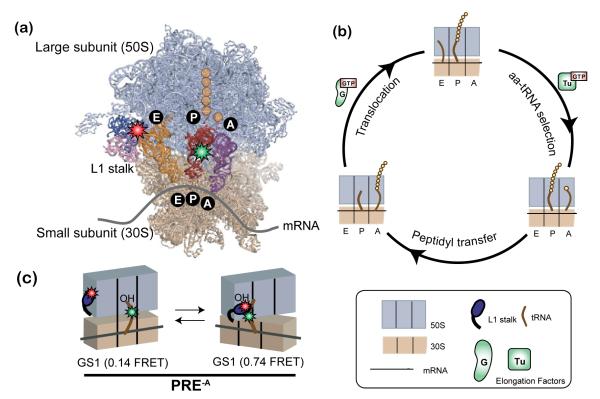
Figure 6.
Ribosome-catalyzed protein synthesis. (a) X-ray crystallographic structure of the ribosome with its mRNA template and tRNA substrates (PDB ID: 2J00 and 2J01) and our smFRET labeling scheme. The 50S ribosomal subunit is shown in lavender and the 30S ribosomal subunit in tan. The L1 stalk consists of 23S rRNA helices 76-78 (pink) and ribosomal protein L1 (dark blue). There are three tRNA binding sites on the ribosome for aminoacyl-tRNA (A site, purple), peptidyl-tRNA (P site, red), and deacylated-tRNA (E site, orange). Individual amino acids are shown as yellow circles. Our smFRET labeling strategy places a Cy3 donor fluorophore (green star) within the central fold, or elbow, domain of the P-site tRNA and a Cy5 acceptor fluorophore (red star) within the L1 protein of the L1 stalk. (b) The three fundamental steps of the translation elongation cycle: aa-tRNA selection, peptidyl transfer and translocation (see text for more detail). (c) The pre-translocation complex analog (PRE-A) used in our temperature-dependence studies. In the absence of EF-G the PRE-A complex fluctuates between two distinct global conformational states, GS1 and GS2 (see text and Supporting Methods for detailed description).