Figure 1. Study Design.
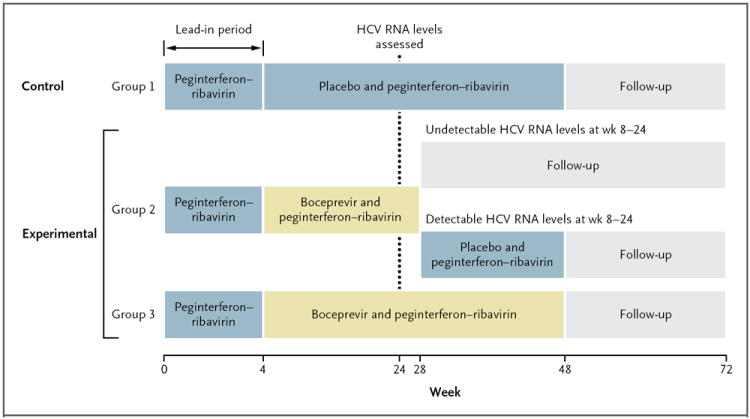
Patients in each of the two study cohorts were randomly assigned to a treatment group in a 1:1:1 ratio. All patients received peginterferon alfa-2b–ribavirin during the 4-week lead-in period. Subsequently, patients assigned to group 1 received 44 weeks of peginterferon alfa-2b–ribavirin as well as a placebo capsule; patients assigned to group 3 received peginterferon–ribavirin as well as boceprevir for 44 weeks; and patients assigned to group 2 received peginterferon–ribavirin as well as boceprevir for 24 weeks, and those with a detectable hepatitis C virus (HCV) RNA level at any visit between weeks 8 and 24 received peginterferon–ribavirin plus placebo from week 28 to week 48. Treatment was discontinued for reasons of futility if the HCV RNA level was detectable at the week 24 visit. Boceprevir was given for a total of 24 weeks in group 2 (irrespective of the rapidity of the decrease in the viral load) and, unless futility had been shown, for a total of 44 weeks in group 3. The x-axis numbers are not to scale.
