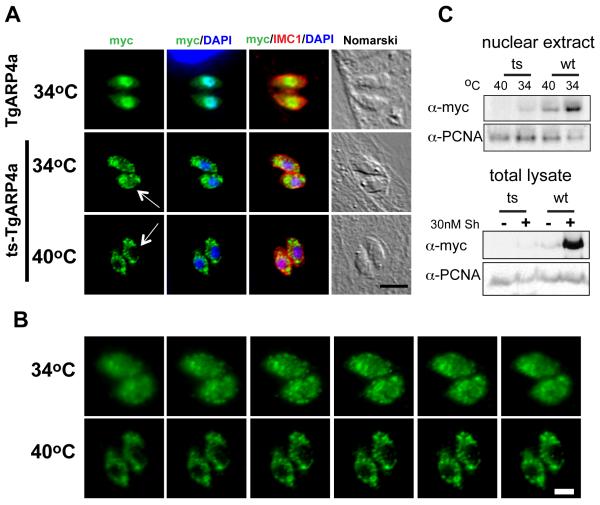
Figure 5. The I621T mutation in tsTgARP4a results in mis-localization.
(A) In order to better understand the temperature defect caused by tsTgARP4a, we compared the subcellular localization of this mutant protein to the wild type isoform over expressed in the parental strain. Transgenic clones expressing DDmyc-wtTgARP4a showed tight nuclear localization at either temperature (34°C expression shown only in top panels); green=anti-myc stain. By contrast, DDmyc-tsTgARP4a was primarily cytoplasmic with some nuclear localization at the non-permissive temperature of 34°C (middle panels). Fluorescent micrographs of DDmyc-tsTgARP4a transgenic parasites were taken using >10-fold longer time exposures. Magnification bar (5 μm) is indicated. (B) Image series scanning through representative 34°C and 40°C vacuoles demonstrated the absence of detectable nuclear DDmyc-tsTgARP4a at the non-permissive temperature. Magnification bar (2 μm) is indicated. (C) Upper panels: To assess the affect of temperature on the levels of nuclear ts or wtTgARP4a, the transgenic clones were induced with 30nM Shield1 at 34°C or 40°C and nuclear extracts prepared as described in the Material and Methods. Nuclear levels of the fusion proteins were detected by Western blots stained with anti-myc antibody. Note the levels of either TgARP4a isoform are affected by higher temperature with the tsTgARP4a undetectable in the parasite nuclear extracts at 40°C similar to the IFA results above. Lower panels: Protein levels in total lysates of transgenic parasites expressing DDmyc-wtTgARP4a or DDmyc- tsTgARP4a fusion proteins grown in the presence or absence of 30 nM Shield1 (Sh) at 34°C were subjected to the western blot analysis using anti-myc antibody. The Western blot membrane was secondarily probed with anti-PCNA1 antibody to ensure equal loading.