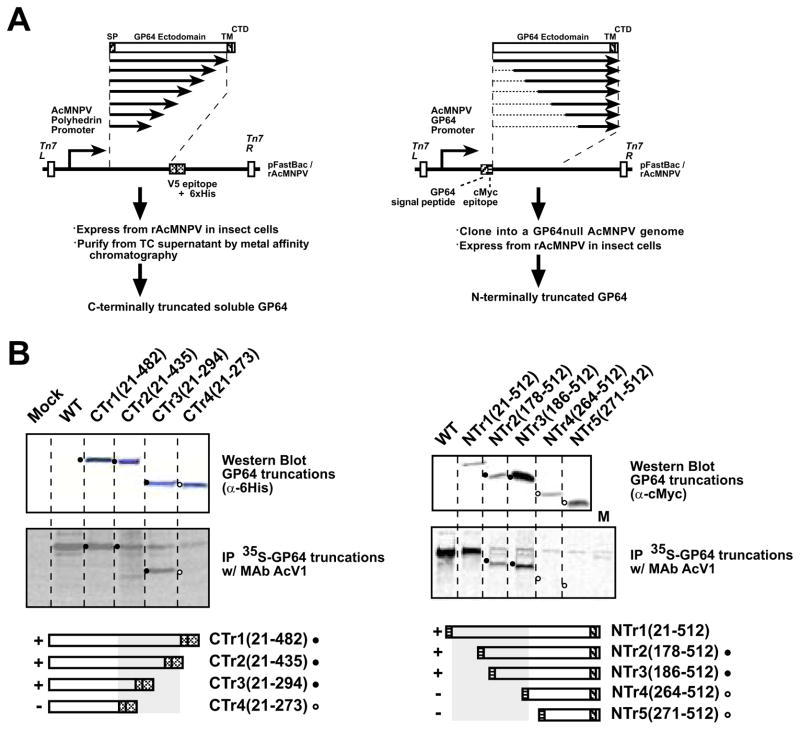
FIGURE 2.
A. Construction of N- and C-terminally truncated GP64 proteins. The strategy for PCR amplification, subcloning, and expression of portions of the GP64 coding region are shown as diagrams. The strategy for construction of C-terminally truncated constructs is shown on the left, and that for construction of N-terminally truncated constructs on the right
B. Western blot analysis and immunoprecipitation of C- and N-terminally truncated GP64 proteins
(SP, signal peptide; cMyc, cMyc tag; V5, V5 epitope; 6His, 6His tag; TM, transmembrane domain; CTD, cytoplasmic tail domain)
Left panel: Cells were infected with recombinant AcMNPV expressing secreted soluble C-terminally truncated GP64 constructs (CTr1(21-482), CTr2(21-435), CTr3(21-294) and CTr4(21-273)). Cells were metabolically labeled with 35S-Methionine for 8 hours and labeled soluble GP64 proteins were harvested from cell supernatants at 72 h pi and prepared as describe in the Materials and Methods section. GP64 was immunoprecipitated with MAb AcV1 and examined by SDS-PAGE (center panel). The top panel shows a Western blot of the cell supernatant preparations used for Immunoprecipitation experiments. GP64 constructs were detected on Western blots with an anti-6His antibody. Circles (filled or open) represent the positions of corresponding bands in upper and center panels. Open circles represent the positions of proteins that were not immunoprecipitated by AcV1 (center panel). Grey boxes (bottom panels) indicate constructs immunoprecipitated by AcV1 and containing the AcV1 epitope
Right panel: Cells infected with wild type or recombinant AcMNPV constructs and expressing wild-type AcMNPV GP64 or recombinant N-terminally truncated GP64 constructs (NTr1(21-512), NTr2(178-512), NTr3(186-512), NTr4(264-512) and NTr5(271-512)) were metabolically labeled with 35S-Methionine for 8 hours and cells were lysed at 72 h pi. GP64 was immunoprecipitated with MAb AcV1 and examined by SDS-PAGE (center panel). The top panel shows a Western blot of extracts used for Immunoprecipitation experiments. On Western blots, GP64 constructs were detected with an anti c-Myc antibody.