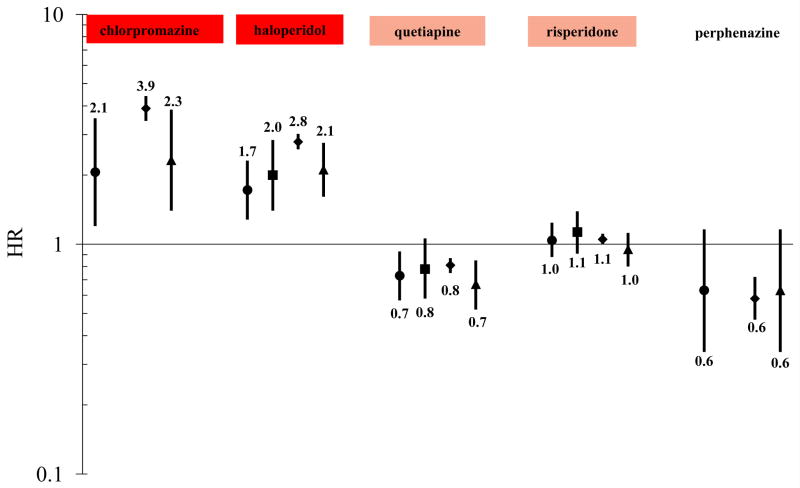
Figure 2.
Change-in-estimate adjusted* HRs for sudden cardiac death/ventricular arrhythmia, fatal sudden cardiac death/ventricular arrhythmia, death, and cardiovascular death—each versus olanzapine as the reference exposure†
Outcome key: ● SD/VA ■ Fatal SD/VA ◆ Death ▲ Cardiovascular death
*SD/VA model adjusted for age, sex, race, state of residence, nursing home residence, prior diagnosis of organic psychosis, and current exposure to cyclic and related antidepressant; Fatal SD/VA model adjusted for age, sex, race, state of residence, and nursing home residence; Death model adjusted for age, sex, race, state of residence, nursing home residence, prior diagnosis of heart failure/cardiomyopathy, prior diagnosis of kidney disease, prior diagnosis of organic psychosis, prior diagnosis of other psychoses, loop diuretic drug marker of disease, and oral corticosteroid drug marker of disease; Cardiovascular death model adjusted for age, sex, race, state of residence, nursing home residence, prior diagnosis of organic psychosis, and current exposure to cyclic and related antidepressant.
†The color-shading is reflective of CredibleMeds ratings of drugs’ QT-prolonging/TdP-inducing potentials, and is consistent with their coloring scheme: a) red = drugs with a risk of TdP; b) pink = drugs with a possible risk of TdP; and c) not shaded = drugs not rated. Of note, olanzapine was transitioned from a non-rated drug to a drug with possible risk (i.e., pink) on 01/04/2013.