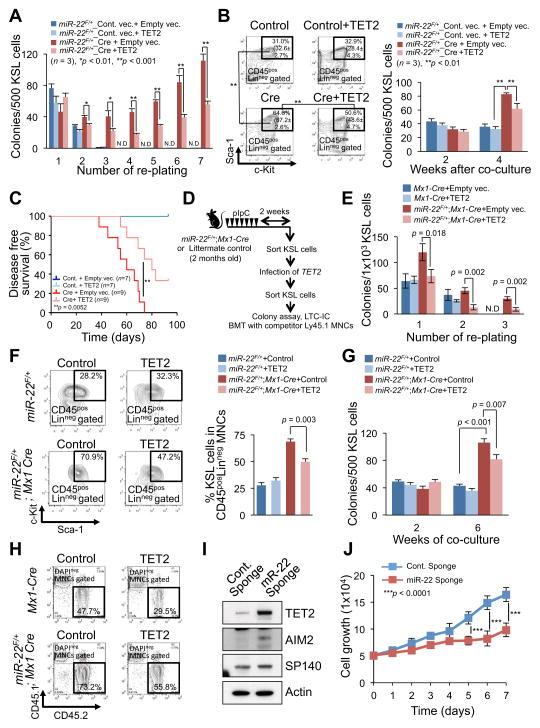
Figure 6. miR-22–TET2 regulatory network affects hematopoietic stem cell function and hematological transformation.
(A and B) Ectopic expression of TET2 reduces the colony forming capacity of miR-22 expressing hematopoietic stem/progenitor cells. The sorted KSL cells from miR-22F/+ mice were infected with Cre-GFP or GFP control vector. GFP+KSL cells were resorted and infected with empty vector or TET2 expressing lentiviral particles. Cells were then incubated in semi-solid medium. Colony replating assay was performed and the resulting colonies were counted at the indicated replatings (n=3) (A). Some of TET2 infected cells were also subjected to LTC-IC assay. Representative flow cytometry data of the positivity of c-Kit/Sca-1 in LinnegCD45pos gated cells 2 weeks after co-culture with stromal cells and mean percentages ± S.D. of KSL cells in Linneg CD45pos (in brackets) are shown (n=3) (B, left). Colony forming capacities were also determined at the indicated weeks after co-culture with stromal cells (n=3) (B, right).
(C) TET2 attenuates the hematopoietic malignancies induced by miR-22 overexpression. KSL cells sorted from miR-22F/+ mice were infected with Cre-GFP or GFP control vector followed by TET2 infection, and 1500 GFP+KSL cells were transplanted into recipient mice with 2.0×105 Ly45.1 competitor bone marrow mononuclear cells (BM MNCs). Disease free survival of recipient mice was examined by Kaplan-Meier survival curves. Log-rank test was used to generate P-value.
(D) Overview of the experimental design for introduction of TET2 into KSL cells from miR-22 transgenic mice. KSL cells purified from miR-22 transgenic mice were subjected to infection with TET2 expressing vector, and their characteristics were evaluated in vivo and in vitro.
(E) Colony forming capacity of miR-22 transgenic progenitor cells is attenuated by ectopic expression of TET2. 2 weeks after pIpC administration, sorted KSL cells from miR-22F/+;Mx1-Cre mice were infected with TET2 expressing vector and resorted KSL cells were then subjected to the incubation in semi-solid medium. The resulting colony numbers were scored in three independent littermate pairs in the indicated platings (n=3). N.D., non-detectable.
(F and G) Ectopic expression of TET2 causes a reduction in LTC-IC capacity of miR-22 transgenic progenitor cells. TET2 infected KSL cells from miR-22F/+;Mx1-Cre mice or littermate controls, as shown in Figure 6D, were co-cultured with stromal cells for the indicated weeks. Maintained KSL cells were evaluated 2 weeks after co-culture (n=3) (F). At the indicated times after co-culture, the capacity of colony formation was also determined in semi-solid medium (n=3) (G).
(H) Increased reconstitution capacity of KSL cells of miR-22 transgenic mice is reduced by ectopic expression of TET2. The sorted KSL cells from miR-22F/+;Mx1-Cre mice or littermate controls were infected with TET2 expressing vector. 1×104 KSL cells were resorted and subjected to the bone marrow transplantation with 5×105 competitor BM MNCs. Representative flow cytometry data of the CD45.1/CD45.2 positivity at 6 weeks after transplantation are shown.
(I) Inhibition of miR-22 increases the expression of TET2 and its putative target genes in human leukemic cells. K562 cells were infected with the vector encoding miR-22 sponge or control sponge, and 48 hrs after the infection cell lysates were subjected to immunoblot analysis for the indicated proteins.
(J) Inhibition of miR-22 suppresses the proliferation of human leukemic cells. K562 cells were infected with the vector encoding miR-22 sponge or control sponge. 48 hrs after puromycin selection, 5×104 cells were incubated for 7 days. Optical densities of the cells were determined at the indicated times (n=3). All error bars indicate ± S.D. “see also Figures S4G–S4K and S5”.