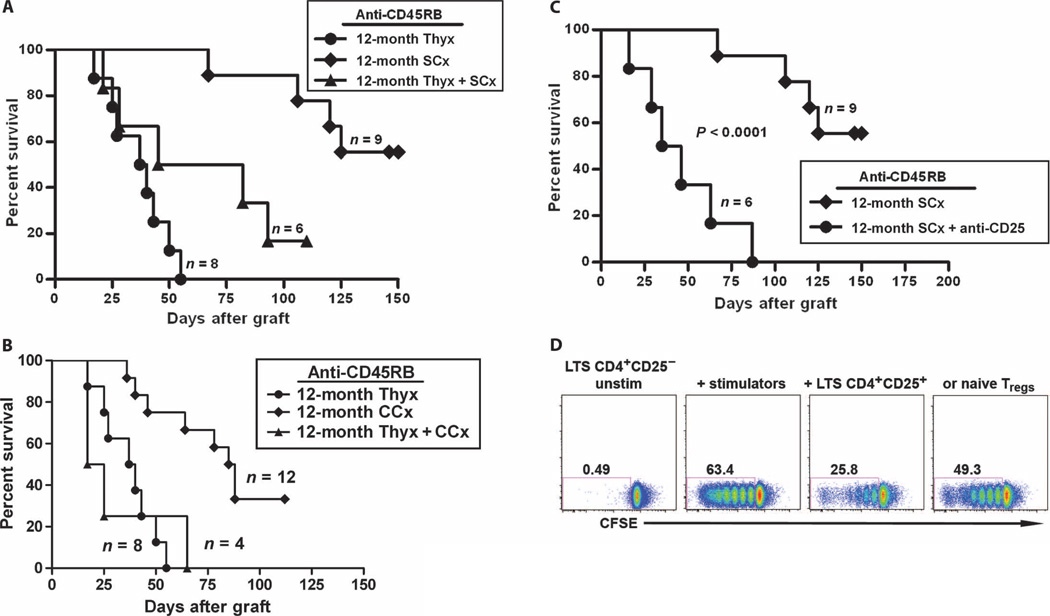
Fig. 6.
Tolerance-rejuvenating effect of castration on the survival of cardiac allografts in anti-CD45RB–treated aged mice was thymus- and Treg-dependent. Twelve-month-old mice underwent surgical or chemical castration with or without thymectomy. (A and B) Thymectomy was performed 1 month before cardiac allotransplantation. Thymectomy abrogated the effect of both surgical (A, P < 0.05 for surgical castration versus thymectomy/surgical castration) and chemical (B, P < 0.005 for chemical castration versus thymectomy/chemical castration) castration on restoring tolerance induced by anti-CD45RB. (C) Castration-mediated tolerance was Treg-dependent. Recipients are treated with 250 µg of anti-CD25 intraperitoneally on days −6 and −1. CD4+CD25+ T cell depletion was verified on day 10. Depletion of Tregs resulted in a significant decrease in heart allograft survival (MST > 128.8 ± 28.2 days versus 46.0 ± 25.6 days, anti-CD25–treated, P < 0.0001). (D) Suppressive effect of Tregs from the castrated, aged long-term survivor (LTS) on native T cell response to alloantigen. To assess the function of Tregs in the castrated recipients of cardiac allograft, we used an in vitro mixed lymphocyte reaction assay. CFSE-labeled CD4+CD25− T effector cells from LTS B6 mice alone (left), or stimulated with C3H splenocytes (middle left), in the presence of CD4+CD25+ T cells from either LTS (middle right) or naïve B6 (right). Tregs from the LTS mice (3:2 responders/Tregs). Data represent one experiment performed in duplicate.