Fig. 3.7.
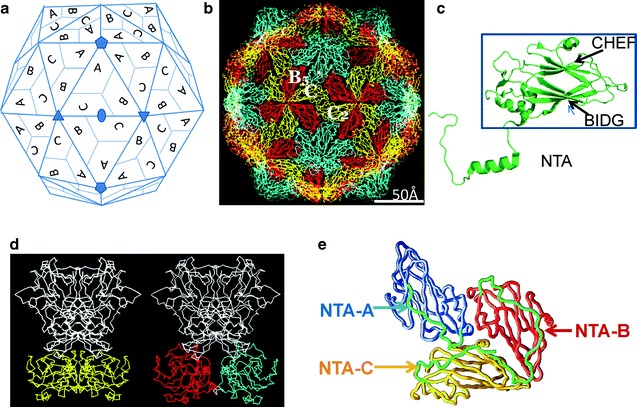
Subunit organization and conformational switching in T = 3 icosahedral viruses. (a) T = 3 lattice as viewed along the icosahedral twofold axis. A set of five-, three-, and twofold axes of the T = 3 icosahedron are denoted. The three quasi-equivalent “subunits” in the asymmetric unit of the T = 3 lattice are indicated by A, B, and C. In a T = 3 structure, these subunits are chemically equivalent. Application of the icosahedral symmetry generates 60 sets of these quasi-equivalent subunits. Disposition of the symmetry-related subunits is also indicated. The formation of the rings of 5 and 6 is clearly seen. The A subunits cluster around the fivefold axis, whereas B and C alternate around the six-coordinated positions. (b) The packing of the canonical trapezoid-shaped β-barrel domains as typically observed in a T = 3 icosahedral virus structures. The view is along the icosahedral twofold axis, same as in (a). The β-barrels corresponding to the quasi-equivalent A, B, and C in (a) are colored in cyan, red, and yellow, respectively. The icosahedral shell can be considered as built from 60 A/B to 30 C/C dimers. The A/B dimers are related by local twofold symmetry (AB5 in the figure), and C/C dimers are related by icosahedral symmetry (C/C2 in the figure). (c) A typical jelly roll β-barrel domain [taken from the structure of San Miguel sea lion virus (Chen et al. 2006), an animal calicivirus] that participates in the T = 3 icosahedral shell. The β-barrel motif (inside the box) consists of eight β-strands organized into two twisted antiparallel β-sheets generally referred to as BIDG and CHEF (Rossmann and Johnson 1989). The letters refer to the position of β-strands in the primary sequence: B is the most N-terminal, and H is the most C-terminal. An N-terminal arm that projects inward from the β-barrel participates in the intersubunit interactions. (d) Comparison of the C/C2 (left) and A/B5 (right) dimers in the Norwalk virus T = 3 structure (Prasad et al. 1999) showing the flat and bent conformations, respectively. In Norwalk virus, the capsid protein consists of two domains: the shell domain (S), with a β-barrel motif, and a protruding domain (P). Only the S domain participates in the shell contacts. The β-barrel domains of A, B, and C are colored as in (b). The P domain which participates in the dimeric interactions is colored in white. Although structurally not similar, capsid protein of tombus viruses, such as TBSV, also has a protruding (P) domain (Harrison et al. 1978). (e) Nonequivalent interactions, as viewed from inside of the capsid, between the NTAs of the ABC subunits that are observed in the Norwalk virus structure, shown here for example (see text). The NTAs (green) from different subunits are marked. The ABC subunits are shown using the same color scheme as in (b)
