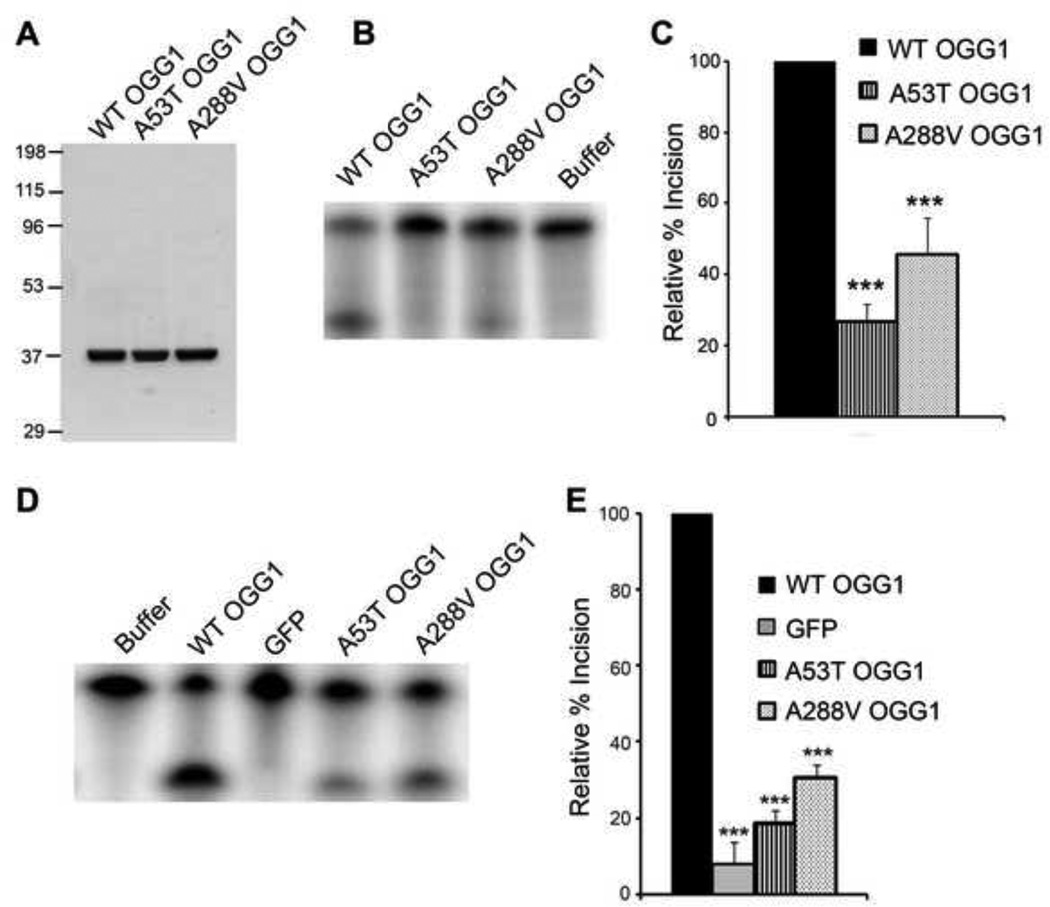
Fig 1. Decreased incision activity of OGG1 polymorphisms.
(A) The indicated OGG1 recombinant proteins were expressed in E. coli, purified using a Ni-NTA column, followed by a thrombin cleavage step and subsequent FPLC purification. Gel Code Blue staining of the wild-type (WT), A53T, and A288V recombinant proteins (2.5µg). (B) OGG1 recombinant proteins (64 nM) were incubated with a 5′-end-labeled oligonucleotide duplex containing an 8-oxoG lesion. After a 15 minute incubation at 37°C, the samples were run on a 15% polyacrylamide gel containing 7 M urea and imaged by a phosphorimager. A representative experiment is shown. (C) The histogram represents the mean ± S.E.M. from nine independent experiments. The percent incision was calculated by taking the amount of cleaved substrate (lower band) normalized to the amount of cleaved + uncleaved substrate (lower + upper bands). The data were normalized to the incision activity of OGG1 alone (100%). (D) Nuclear extracts from OGG1−/− MEFs transfected with WT or polymorphic GFP-tagged OGG1 were reacted with a 5′-end-labeled oligonucleotide duplex containing an 8-oxoG:C mispair as in B. A representative experiment is shown. (E) The histogram represents the mean ± S.E.M. from 5 independent experiments. Percent incision was calculated as in C. ***p< 0.001 comparing WT and variant forms of OGG1 using one-way ANOVA and Tukey's post-hoc test.