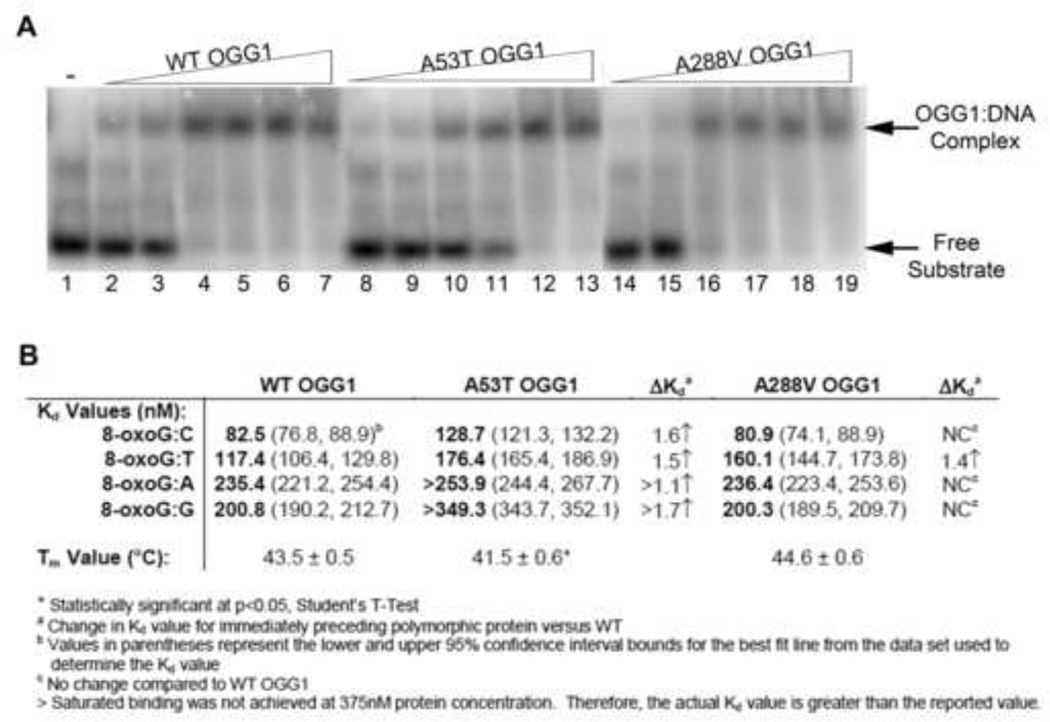
Fig. 3. Binding of WT and polymorphic OGG1 proteins to 8-oxoG:C substrate.
(A) Increasing concentrations of recombinant OGG1 proteins were incubated with 33.3 nM 5′-end-labeled oligonucleotide duplex containing an 8-oxoG lesion paired with A, T, C, or G for 5 minutes on ice. The bound complexes were separated from free probe on a 6% native polyacrylamide gel and imaged on a phosphorimager. A representative experiment for the 8-oxoG:C substrate is shown. The presence of the higher band indicates the formation of the protein:substrate complex. Lane 1: Buffer alone. Lanes 2, 8, 14: 32 nM protein. Lanes 3, 9, 15: 64 nM protein. Lanes 4, 10, 16: 125 nM protein. Lanes 5, 11, 17: 190 nM protein. Lanes 6, 12, 18: 250 nM protein. Lanes 7, 13, 19: 375 nM protein. (B) Dissociation constants calculated from EMSAs and melting temperatures from thermal stability assays for wild-type and polymorphic OGG1 proteins (see Suppl. Fig. 1–3 for graphs used for calculations).