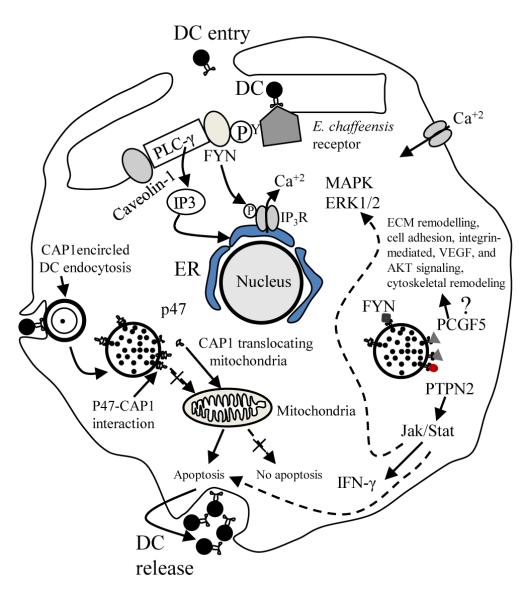
Figure 4.
A proposed model of TRP47 effector function in E. chaffeensis infection of the macrophage based on defined molecular interactions study and previous reports (Refs. 106,107,82,108,109). Binding of E. chaffeensis to its receptor directly or indirectly activates FYN. Activated FYN tyrosine kinase phosphorylates and activates PLC-γ, which hydrolyzes membrane phospholipid PIP2 (phosphatidylinositol biphosphate), resulting in increased level of IP3 (inositol 1,4,5 triphosphate) and release of Ca2+ from intracellular stores, and Ca2+ influx. FYN may regulate the function of IP3 receptor by phosphorylation and promoting release of Ca2+ from the endoplasmic reticulum. FYN also phosphorylates caveolin-1 involved in ehrlichial entry. TRP47-PTPN2 interaction modulates cytokine signaling events by exerting negative feedback on the Jak/Stat pathway by dephosphorylation of Jaks and Stats involved in intravacuolar maintenance and survival of E. chaffeensis. TRP47-PCGF5 interaction modulates gene transcription associated with cell signaling and remodeling of cytoskeleton facilitating and supporting intracellular survival of E. chaffeensis. CAP1 promotes rapid actin dynamics in conjunction with ADF/cofilin and is required for cell morphology, migration, and endocytosis. Mitochondrial shuttling of CAP1 promotes actin- and coflin-dependent apoptosis, which may play important role in release of DC ehrlichiae from the monocyte (Reproduced with permission from the American Society for Microbiology, license 2541950462120).