Fig. 3.
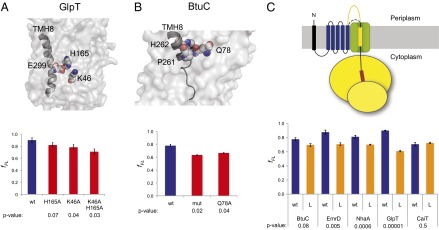
Interacting residues in N-terminal TMHs and the length of the loop preceding the C-terminal TMH affect the pulling force exerted on C-terminal TMHs. (A) Surface representation of the GlpT crystal structure, with E299 in TMH8 and interacting residues K46 and H165 shown as Corey-Pauling-Koltun (CPK) models. The red bars show the fFL for mutants H165A, K46A, and K46A+H165A. wt is the nonmutated GlpT TMH1-TMH(i+1) construct (blue bar, Fig. 2B). (B) Same as in A, but for BtuC. Residues P261 and H262 in TMH8, together with the interacting residue Q78, are shown as CPK models. (C) fFL for TMH1-TMH(i+1) constructs in which the loop preceding the C-terminal test TMH(i+1) was lengthened by 85 residues (L and orange bars). wt indicates results for the nonmutated constructs (blue bars, Fig. 2B). SEs are shown (number of independent measurements ≥3) and P values with respect to the nonmutated (wt) constructs are indicated.
