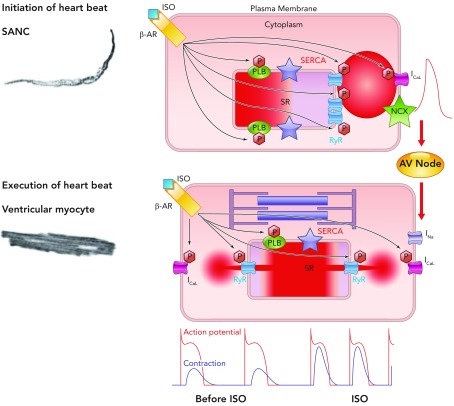
FIGURE 10.
A general theory of cardiac function, involving similar mechanisms in SANC and ventricular myocytes
Roughly periodic diastolic Ca2+ releases in the form of LCRs in SANC interact with sarcolemmal NCX to produce an inward current that increases the rate of diastolic depolarization toward the threshold potential for activation of L-type Ca2+ channels. Ca2+ influx through these channels depolarizes SANC and activates RyRs to produce en masse emptying of the SR in a process known as CICR and leads to the action potential-induced Ca2+ transient. Depolarization of SANC spreads via cell-to-cell contacts, leading to depolarization of atrial myocytes and, following conduction via the atrioventricular node, to depolarization of ventricular myocytes. This electrical process involves influx of Na+ ions into ventricular myocytes via fast sodium channels. Depolarization subsequently causes influx into ventricular myocytes of Ca2+ via L-type Ca2+ channels, which again activate SR-based RyRs, leading to en masse emptying of the SR of its Ca2+, which binds to the sarcomere to cause shortening and the ultimate goal of cardiac inotropism. In this schema, the chemical signal of Ca2+ release from the SR in SANC initiates an electrical signal, which is passed to ventricular myocytes. In ventricular myocytes, electrical depolarization then initiates a chemical signal (intracellular Ca2+ release) to produce the ultimate goal: a timely and efficient mechanical oscillation, i.e., a heart beat. Thus heart rate (chronotropy) controlled by SANC leads to execution of a heart beat by ventricular myocytes by utilizing an almost identical set of molecules behaving in the same, albeit differently ordered, manner, a general theory of cardiac function. Increasing or decreasing the rate of activity of SANC in turn increases or decreases the force of contraction of ventricular myocytes via phosphorylation/dephosphorylation of the same molecules (phospholamban, RyR, L-type Ca2+ channels, and delayed rectifier K+ channels) via the same mechanisms involving PKA and CAMKII in these two neighboring yet physiologically diverse cell types (see supplemental movie at Physiology's website; the movie was created by Victor Maltsev).