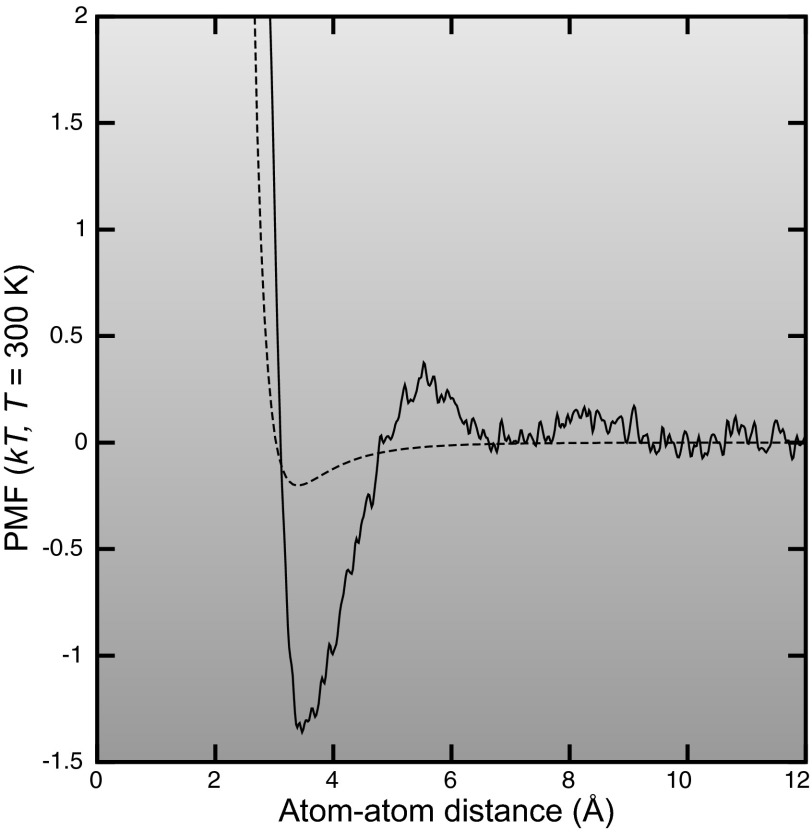
Figure 1.
The potential of mean force between two neutral carbon atoms in the presence of water, calculated using 10 ns of metadynamics simulation. The dotted line shows the bare Lennard-Jones potential for comparison. The Lennard-Jones parameters are those of a carbonyl or guanadinium carbon atom, type C in the CHARMM27 force field. Since both atoms are neutral, the only direct forces between them are van der Waals forces. The presence of water is seen to greatly modify these van der Waals interactions, due to the hydrophobic effect and the varying effect of van der Waals forces between the atoms and water molecules as the atoms approach each other.