Abstract
Purpose
Osteocalcin (OC) is a protein constituent of bone matrix and a marker of bone formation. We characterized the heritability of serum OC measures and identified genomic regions potentially involved in the regulation of OC via high-density genome-wide linkage analysis in African ancestry individuals.
Methods
African ancestry individuals (n=459) were recruited, without regard to health status, from seven probands (mean family size = 66; 4,373 relative pairs). Residual heritability of serum OC measures was estimated and multipoint quantitative trait linkage analysis was performed using pedigree-based maximum likelihood methods.
Results
Residual heritabilities of total OC, uncarboxylated OC, carboxylated OC and percent uncarboxylated OC were: 0.74±0.10, 0.89±0.08, 0.46±0.10 and 0.41±0.09, respectively. All OC measures were genetically correlated with whole body bone mineral content (BMC). We obtained strong evidence of bivariate linkage for percent uncarboxylated OC and whole body BMC on chromosome 17 (LOD=3.15, 99cM).
Conclusions
All forms of OC were highly heritable and genetically correlated with total body BMC in these African ancestry families. The identified linkage region contains several candidate genes for bone and energy metabolism including COL1A1 and TNFRSF11A. Further studies of this genomic region may reveal novel insight into the genetic regulation of OC and bone mineralization.
Keywords: osteocalcin, genome-wide linkage, African ancestry, bone mineral
INTRODUCTION
Osteocalcin (OC), a major protein constituent of bone matrix that is synthesized by osteoblasts, is considered a biomarker of bone formation and bone turnover [1, 2]. Circulating OC levels are associated with age, sex, body size and hormone replacement therapy but are also under strong genetic control [3-8]. Candidate gene studies, conducted primarily among Caucasians, have documented association between several loci and serum OC concentrations including the OC structural locus (BGLAP) [9] and genes encoding the vitamin-D receptor (VDR) [10], calcitonin receptor (CALCR) [11], Klotho (KL) [12] and transforming growth factor beta 1 (TGFB1) [13]. Only one genome-wide linkage study of serum OC concentration has been conducted in humans. That study detected suggestive linkage on chromosomes 16 and chromosome 20 in Mexican Americans [3]. Quantitative trait locus (QTL) linkage analysis in baboons has also identified an orthologous region on human chromosome 6p23-21.3 for serum OC [5, 6].
A limitation of previous studies of OC is that they have focused on measures of total serum OC. However, OC undergoes γ-carboxylation at its three glutamic acid residues. γ-carboxylation is dependent on vitamin K, such that individuals deficient in vitamin K have a higher percent of their total OC as uncarboxylated OC (ucOC) [14]. Since there are physiologic differences between the carboxylated and uncarboxylated forms of OC, it is important to elucidate potential differences in their determinants. Furthermore, no genetic studies of OC to date have included African ancestry individuals, who have significantly lower OC levels compared with Caucasians [15, 16]. Therefore, in this study we determined the correlates and heritability of serum total, carboxylated, and uncarboxylated OC in 459 individuals who were members of seven large, multi-generational families of African ancestry. We also performed genome-wide linkage analyses using a high-density polymorphism panel in order to identify potential QTL that may influence OC levels in this understudied population group.
MATERIALS AND METHODS
Study Sample
Participants for this analysis were from the Tobago Family Health Study. The Tobago population is predominately of West African origin with low admixture [17]. Briefly, probands were recruited without regard to their medical history from a cohort study of bone mineral density and body composition on the Caribbean island of Tobago [18]. Probands were eligible if they had a spouse willing to participate and had at least six living offspring and/or siblings aged ≥18 years and who were residing in Tobago. All first-, second-, and third-degree relatives of the probands and their spouses were invited to participate. The final set of 471 individuals comes from 7 extended families consisting of: 21, 26, 28, 49, 96, 98 and 153 individuals. Out of the total study, there were 459 individuals with OC measures who form the basis of the current analysis (278 women, 181 men; mean family size 66; 4,373 relative pairs). Written informed consent was obtained from each participant. The Tobago Division of Health and Social Services and the University of Pittsburgh Institutional Review Boards approved this study.
Osteocalcin Measures
Total OC, ucOC and percent ucOC (%ucOC) were measured in fasting, morning serum samples. Serum was frozen at −80°C until analysis. Serum concentrations of total and uncarboxylated OC were measured, in duplicate, in previously unthawed specimens by radioimmunoassay, as previously described elsewhere [19]. Briefly, carboxylated and ucarboxylated OC are measured in serum using a radioimmunoassay utilizing an antibody made against OC purified from human bone. This antibody recognizes both carboxylated and uncarboxylated OC equivalently. Carboxylated OC is separated from uncarboxylated OC by adsorption on hydroxyapatite. After adsorption of serum onto a standard suspension of hydroxyapatite, the supernatant contains uncarboxylated OC. Total OC is measured in the original serum and ucOC is measured in the supernatant. The two metabolites are separated using absorption on hydroxyapatite. Carboxylated osteocalcin (carbOC) was calculated as (total OC – ucOC) and %ucOC was calculated as (ucOC/totalOCx100%). Intra-assay variations were 4.8% and 7.1% for total OC and ucOC, respectively. Inter-assay variation ranged from 3.5-5.9% for total OC and was 8.2% for ucOC.
Dual-energy X-ray Absorptiometry
Whole body bone mineral content (BMC, grams) and density (BMD, g/cm2) were measured by DXA using the array beam mode on a Hologic QDR 4500W scanner (Hologic, Inc.; Bedford, MA). Standardized procedures for participant positioning and scan analysis were followed according to the manufacturer's recommended protocol. Scans were analyzed with QDR software version 8.26a. The short-term in vivo precision of the DXA measurements was assessed in 12 subjects. All CVs were less or equal to 1.16% and all rest-retest correlations are above 0.99. A phantom was scanned daily and reviewed to maintain longitudinal quality assurance of the scanner during the course of the study.
Other Measurements
Body weight was measured to the nearest 0.1 kg on a balance beam scale. Standing height was measured to the nearest 0.1 cm, without shoes, using a wall-mounted stadiometer. Body mass index was calculated as weight in kilograms divided by standing height in meters2.
Demographic, lifestyle and medical history variables were collected by trained clinic staff through administration of a questionnaire and interview. Race was self-reported based on grandparental ethnic origin. Smoking status was classified as either current or not (yes/no), and participants reporting ever smoking <100 cigarettes in their lifetime were considered non-smokers. Alcohol consumption was assessed by questionnaire and is coded based on having >1 drink per week (yes/no). Physical activity was assessed by the number of minutes walked per week and participants were dichotomized into “not active” (≤25 minutes walked/week) or “active” (>25 minutes walked/week). Participants were asked to bring current medications to their interview, and staff recorded each medication. Diabetes was defined as a fasting glucose level >126 mg/dl or current use of diabetes medication. Hypertension was defined as a seated diastolic blood pressure >90 mmHg, systolic pressure >140 mmHg and/or current use of anti-hypertensive medication.
Reproductive characteristics collected included postmenopausal status, parity, use of oral contraceptives and hormone replacement therapy (HRT). Because only 5 women reported using HRT, this variable was not included in this analysis. Women were considered postmenopausal only if they had no menses for at least 12 months and were >40 years old, or if they had a hysterectomy or ovariectomy.
Genotyping and Multipoint Identity-By-Descent (IBD) Calculation
Genomic DNA was isolated from whole blood extracted by the salting out method and isolated by a Qiagen column procedure (Qiagen, Santa Clara, CA). Whole-genome genotyping by fluorescence-based methods was performed using the Infinium HumanLinkage-12 Genotyping BeadChip (Illumina, San Diego, CA). After excluding single nucleotide polymorphisms (SNPs) with call rate <90%, Hardy-Weinberg equilibrium (P<0.001), minor allele frequency <0.05 or multipoint IBD calculation incompatibility, we retained 1512 autosomal SNPs and used the Markov chain Monte Carlo algorithm as implemented in the program Loki [20] to calculate multipoint IBD. The final SNP set had a median MAF of 0.325 with a median spacing of 1.92 cM based on the Kosambi mapping function [21].
Statistical Analysis
All traits were assessed for non-normality and transformed as necessary. Outliers, defined as ≥4 SD from the mean, were removed for each trait; no more than eight observations were removed from any OC trait. To determine significant correlates for each OC trait, we tested each covariate separately in an age and sex adjusted model; age and sex were forced into each model because they are known correlates of serum OC concentrations. Because we analyzed family data, we used the variance component framework as implemented in the program SOLAR [22], which accounts for the non-independence between observations by using a familial correlation matrix. All potentially significant covariates were then assessed simultaneously for each trait; we required a p-value ≤ 0.05 for inclusion in our final models.
Maximum likelihood methods were used to simultaneously model the effect of additive genetics, or heritability (h2), fixed covariate effects and error. Heritability reported herein is the residual heritability (h2r), which is estimated as the proportion of phenotypic variation after the effects of covariates have been removed.
To compare the effects of covariates across all traits, we calculated the percent difference in the OC related trait per unit increase in covariate. Percent differences were calculated as (beta coefficient*unit/mean trait value)*100. For continuous variables, the unit range was 1 SD, and for dichotomous variables, the unit range was 1.
Multipoint linkage analysis was performed using an extension of the variance components model described above. Maximum likelihood methods were used to estimate the expected variance attributable to a theoretical QTL, based on the expected covariance between relatives, which were estimated using the multipoint IBD probabilities. The model containing the parameter for the theoretical QTL was then compared with models incorporating only polygenic effects using a likelihood ratio test (LRT). Logarithm of the odds (LOD) scores, computed as the log10 of the likelihood ratio, were used to assess the significance of the test. By convention, to account for multiple testing LOD score thresholds of 3.0 and 2.0 were considered to represent nominal genome-wide significant and suggestive evidence for QTLs, respectively. In these families, we have 80% power to detect a QTL with heritability as low as 0.33 at a LOD of 3.0 and heritability as low as 0.29 at a LOD of 2.0.
Secondary to our univariate heritability and linkage analyses, we attempted to refine the OC genomic signal to one that may reflect overall bone status as assessed by dual X-ray absorptiometry (DXA) measures of whole body bone mineral density (wbBMD) and mineral content (wbBMC). We first estimated genetic (ρG), environmental (ρE) and phenotypic (ρ) correlations between OC and the DXA traits using a bivariate extension of the variance components framework [23]. For any OC and bone trait pair that had significant (P<0.05) genetic correlation, we also performed bivariate linkage analysis in the same manner as single trait analysis described above. All models were adjusted for covariates deemed appropriate a priori, including age, sex, BMI and menopausal status. To account for multiple testing, we used LOD score thresholds of 2.0 and 3.0 to signify suggestive and strong evidence of bivariate linkage, respectively. We did not set a significance threshold for the LOD score because bivariate linkage analyses involve a LRT with 2 degrees of freedom, so the conventional univariate thresholds are not appropriate [23] and there has been no significant LOD threshold proposed for bivariate analyses.
RESULTS
Subject Characteristics
Individuals were on average 43 years (range, 18-100 years), and age did not differ by sex (Table 1). Women were significantly heavier than men. Men were more likely to be current smokers, drink at least one alcoholic drink per week and to walk ≥ 25 minutes per week than women (p < 0.05 for all). Both men and women watched about 15 hours of television per week. Women drank more milk and caffeinated beverages and were more likely to use vitamin D and calcium supplements than men (p<0.05 for all). Diabetes and hypertension were prevalent in this population (8.9% and 31.2%, respectively), with treatment prescribed to 75% of diabetics but only 32% of hypertensives. There was no difference in diabetes or hypertension prevalence between men and women (p>0.05). Approximately 32% of women were postmenopausal, 77.3% had at least one child and 33.3% were currently using oral contraceptives. The mean total OC was 4.5±2.9μg/L (range 0.4-15.3 μg/L). About 36% of the total OC was uncarboxylated (mean: 1.55±1.1μg/L; range: 0.2-5.6μg/L). There were no significant differences in unadjusted OC measures between men and women.
Table 1.
Characteristics* of the Afro-Caribbean Families
| Trait | All (n=459) | Men (n=181) | Women (n=278) |
|---|---|---|---|
| Age (years) | 42.6±16.7 | 42.8±17.0 | 42.4±16.4 |
| BMI (kg/m2) | 28.3±6.4 | 26.8±4.9‡ | 29.3±7.0‡ |
| Current Smoker (%) | 4.8(22) | 11.8(21)‡ | 0.4(1)‡ |
| >1 Drink per week (%) | 13.6(62) | 30.6(55)‡ | 2.5(7)‡ |
| Walking >25 min per week (%) | 49.3(224) | 55.6(100)‡ | 45.3(124)‡ |
| Television viewing time (hours/week) | 14.9(8.0) | 16.6(8.5) | 15.5(7.7) |
| Current milk consumption (times/week) | 4.3(2.9) | 3.9(2.9)‡ | 4.6(2.9)‡ |
| ≥1 Caffeine drink per week (%) | 72.5(324) | 66.7(114)‡ | 76.0(209)‡ |
| Current Supplemental Vitamin D use (%) | 18.7(84) | 8.6(15)‡ | 25.0(69)‡ |
| Current Supplemental Calcium use (%) | 14.6(66) | 9.7(17)‡ | 17.8(49)‡ |
| Diabetes (%) | 8.9(39) | 6.6(12) | 9.7(27) |
| Hypertension (%) | 31.2(141) | 30.7(55) | 31.5(86) |
| Post-Menopausal (%) | -- | -- | 31.9(86) |
| Parity (%) | -- | -- | 77.3(215) |
| Current Oral Contraceptive use (%) | -- | -- | 33.3(92) |
| Total Osteocalcin (μg/L) | 4.50±2.91 | 4.58±2.89 | 4.45±2.93 |
| Uncarboxylated Osteocalcin (μg/L) | 1.55±1.08 | 1.52±0.98 | 1.56±1.15 |
| Carboxylated Osteocalcin (μg/L) | 2.92±2.14 | 2.95±2.26 | 2.90±2.07 |
| Percent Uncarboxylated Osteocalcin (%) | 35.87±15.69 | 35.87±15.29 | 35.87±15.97 |
Characteristics are shown as mean±SD or frequency(N)
Comparison by sex is statistically significant (p<0.05)
Association with Covariates and Genetic Heritability Estimates
We assessed the significance of age, sex and other covariate associations with each OC related trait (Table 2). Age was inversely associated with total OC, ucOC and carbOC concentrations such that each 10-year increase in age was associated with a 2.1%-5.1% lower OC concentration (p<0.05 for all). Women had ~10% lower adjusted total OC and carbOC (p<0.01). Each standard deviation (6.4 kg/m2) increase in BMI was associated with a 4.8-5.8% lower concentration of each OC measure (p<0.0001 for all). Postmenopausal women had 17%-28% higher OC measures compared with premenopausal women and men combined (p<0.0001 for all). Prevalence of diabetes was associated with 11.9% and 15.3% lower total OC and ucOC, respectively (p<0.01 for all). The prevalence of hypertension was associated with 9.5% higher ucOC (p=0.007). For every 8 hours of television watched per week, carbOC was 0.4% lower. Current smokers had 17% higher %ucOC (p<0.05) compared with non-smokers.
Table 2.
Covariate Effects* on and Residual Heritabilities of Osteocalcin Related Traits
| Trait | Age (10 years) | Female Sex | BMI (6.4 kg/m2) | Menopausal Status | Diabetes | Hypertension | TV (8 hrs/wk) | Current Smoking | Proportion of Trait Variance |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Covariate Effects (r2) | Genetic Effects (h2r ± SE)§ | |||||||||
| TotalOC | −3.7%¥ | −9.3%‡ | −4.8%¥ | 24.5%¥ | −11.9%‡ | 0.074 | 0.740 ± 0.102 | |||
| UcOC | −2.1%† | −5.0% | −5.7%¥ | 17.3%¥ | −15.3%¥ | 9.5%‡ | 0.074 | 0.893 ± 0.076 | ||
| CarbOC | −5.1%¥ | −9.6%‡ | −5.8%¥ | 27.6%¥ | −0.4%† | 0.084 | 0.456 ± 0.097 | |||
| % UcOC | 3.9%‡ | 1.5% | 17.0%† | 0.031 | 0.407 ± 0.086 | |||||
Values shown depict the percent change in osteocalcin related trait value for each unit change in covariate value. Unit values are shown in parentheses for continuous traits and are 1 for dichotomous traits. Age and sex were forced into all models.
OC: osteocalcin; UcOC: uncarboxylated osteocalcin; CarbOC: carboxylated osteocalcin
Only covariates that showed a consistent, significant association with osteocalcin are shown
All residual heritability estimates had a corresponding p-value < 0.0001
p<0.05
p<0.01
p<0.001
Measured covariates only explained between 3%-8% of the total phenotypic variance in the OC related traits (Table 2). We also found that a large proportion of the phenotypic variance in OC was due to polygenic effects. For example, the residual heritabilities were: 0.74±0.10 for total OC, 0.89±0.08 for ucOC, 0.46±0.10 for carbOC and 0.41±0.09 for %ucOC (p<0.0001 for all).
Genome-Wide Linkage Analysis
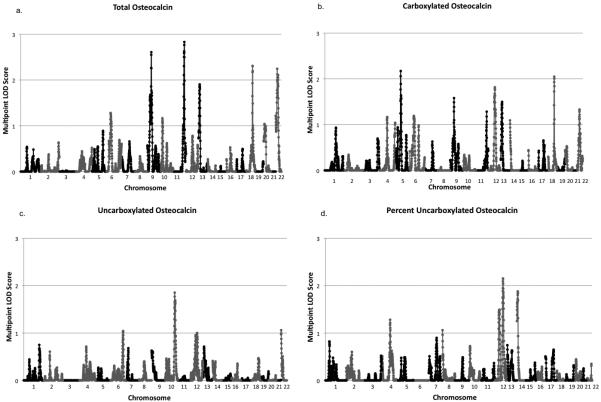
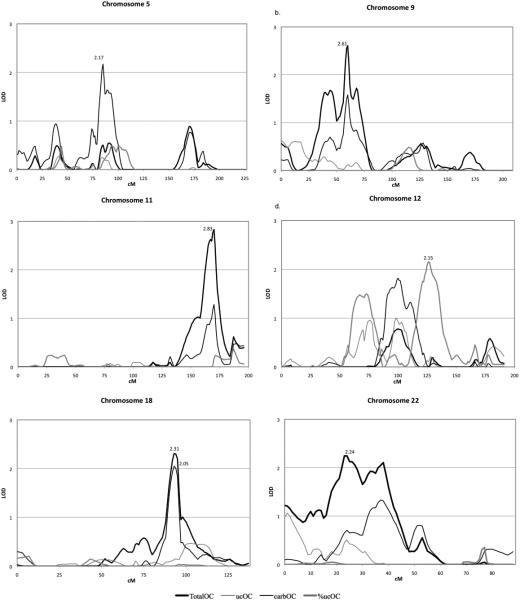
We detected seven regions suggestive of linkage with the OC related traits (Table 3 and Figures 1 and 2). The highest univariate LOD score detected was for total OC at 170cM on chromosome 11 (LOD=2.83; Figure 2c). There were three additional regions suggestive of linkage for total OC: one on chromosome 9 (peak LOD=2.61, 60cM; Figure 2b), another on chromosome 18 (peak LOD=2.31, 93cM; Figure 2e) and the last on chromosome 22 (peak LOD=2.24, 24cM; Figure 2f). We also detected suggestive evidence of linkage for carbOC at the same location as total OC on chromosome 18 (peak LOD=2.05, 93cM; Figure 2e). There was also a unique region on chromosome 5 suggestive of linkage (peak LOD=2.17, 85cM; Figure 2a). Suggestive linkage was also observed on chromosome 12 for %ucOC (peak LOD=2.15, 126cM; Figure 2d). The highest LOD score for ucOC was only 1.85 on chromosome 10 (Figure 1).
Table 3.
Genome-wide Linkage Results for Osteocalcin Related Traits
| Trait* | Peak LOD | QTL Location§ | Genes Under Peak | ||||
|---|---|---|---|---|---|---|---|
| Chrm | cM (range) | Cytogenetic | Position (Mbp) | N | Candidates | ||
| TotalOC | 2.606 | 9 | 60 (57-62) | p13.1-p13.3 | 33.9-38.4 | 69 | none |
| TotalOC | 2.829 | 11 | 170 (162-172) | q24.2-q24.3 | 124.4-130.2 | 76 | none |
| TotalOC | 2.308 | 18 | 93 (89-96) | q21.32-q22.1 | 56.3-59.9 | 25 | TNFRSF11A(RANK) |
| TotalOC | 2.244 | 22 | 24 (17-31) | q12.1-q13.33 | 20.5-25.5 | 111 | MAPK1; MIF |
| CarbOC | 2.172 | 5 | 85 (81-95) | q13.2-q14.1 | 70.7-80.5 | 60 | GDF5-UQCC |
| CarbOC | 2.049 | 18 | 93 (90-97) | q21.32-q22.1 | 56.3-59.9 | 25 | TNFRSF11A(RANK) |
| % UcOC | 2.151 | 12 | 126 (117-136) | q23.1-q24.21 | 96.2-113.2 | 142 | IGF1; TRPV4 |
Only regions with a peak LOD >2.0 are shown.
OC: osteocalcin; %UcOC: percent uncarboxylated osteocalcin; CarbOC: carboxylated osteocalcin
Total OC includes adjustment for age, sex, bmi and menopausal status. Carboxylated OC includes adjustment for age, sex, bmi, menopausal status and TV hours. Percent uncarboxylated OC includes adjustment for age, sex, bmi, menopausal status and smoking.
Location defined by the position of the peak LOD±1.0
Figure 1.
Genome-wide Linkage Results for Osteocalcin Related Traits. This figure shows multipoint LOD scores across chromosomes 1 through 22 for each osteocalcin related trait with at least one LOD score greater than 2.0. Panel a: total serum osteocalcin; Panel b: serum carboxylated osteocalcin; Panel c: serum uncarboxylated osteocalcin; Panel d: serum percent uncarboxylated osteocalcin
Figure 2.
Multipoint LOD Scores for Osteocalcin Related Traits. Chromosomes with at least one peak suggestive of linkage are shown (chromosome 5: a., 9: b., 11: c., 12: d., 18: e., 22: f.). Multipoint LOD scores are plotted by cM location for each OC related trait.
Association of Osteocalcin-related Traits with Whole Body Bone Mineral
Age, sex, BMI and menopausal status explained 34% and 53% of the total phenotypic variance in wbBMD and wbBMC, respectively. Genetic effects were also significant with residual heritabilities of 0.57±0.11 and 0.64±0.10 for wbBMD and wbBMC, respectively (Table 4). A genome-wide scan of these DXA traits identified genomic regions suggestive of linkage on chromosome 3 (wbBMC, 89cM), chromosome 7 (wbBMC, 79cM), chromosome 13 (wbBMC, 85cM; wbBMD, 88cM) and chromosome 15 (wbBMD, 149cM). Analysis of phenotypic correlations showed that higher total OC, carbOC and ucOC were all significantly associated with lower wbBMD; however, only ucOC was inversely associated with wbBMC (P<0.05 for all). There was significant genetic correlation between ucOC and wbBMD (−0.277, P<0.05) and %ucOC with both wbBMD and wbBMC (−0.327 and −0.346, respectively; P<0.05 for both), suggesting that shared genes may influence both traits.
Table 4.
Bivariate Analysis of Osteocalcin and Whole Body Bone Traits
| Trait* | Proportion of Trait Variance | Trait Correlations | QTL (Peak LOD, Chromosome: cM[range$]) | ||||
|---|---|---|---|---|---|---|---|
| Covariate Effects (r2) | Genetic Effects (h2r ± SE)§ | Genetic (ρG) | Environmental (ρE) | Phenotypic (ρ) | Max LOD | Additional LOD>2.0 | |
| Whole Body BMD | 0.337 | 0.573±0.107 | -- | -- | -- | 2.414, 15: 149[134-qter] | 2.114, 13: 88[61-97] |
| TotalOC | -- | -- | −0.204 | 0.011 | −0.131† | -- | -- |
| UcOC | -- | -- | −0.277† | 0.134 | −0.167† | 2.401, 1: 85[83-96] | -- |
| CarbOC | -- | -- | −0.136 | −0.065 | −0.101† | -- | -- |
| % UcOC | -- | -- | −0.327† | 0.230 | −0.045 | 2.676, 17: 102[95-119] |
2.398, 1: 85[82-94] 2.191, 12: 67[61-77] |
| Whole Body BMC | 0.534 | 0.636±0.103 | -- | -- | -- | 2.127, 7: 79[58-85] |
2.079, 3: 89[82-103] 2.079, 13: 85[43-94] |
| TotalOC | -- | -- | −0.040 | −0.066 | −0.047 | -- | -- |
| UcOC | -- | -- | −0.168 | 0.020 | −0.122† | -- | -- |
| CarbOC | -- | -- | 0.040 | −0.080 | −0.014 | -- | -- |
| % UcOC | -- | -- | −0.346† | 0.156 | −0.106 | 3.154, 17: 99[92-120] | 2.156, 2: 271[264-275] 2.108, 12: 67[46-79] |
All models are adjusted for age, sex, BMI and menopausal status
BMD indicates bone mineral density; BMC, bone mineral content.
All residual heritabilities were significant at p < 0.0001
Location is defined as LOD±1.0 when LOD<3.0 and LOD±2.0 when LOD≥3.0
p<0.05
Bivariate linkage analysis identified one region with strong evidence of genome-wide linkage and three regions with suggestive evidence of genome-wide linkage that were not present in any of the univariate linkage results. The maximum LOD score was 3.15 for a QTL influencing %ucOC-wbBMC on chromosome 17 (99cM). This locus was also suggestive of linkage for %ucOC-wbBMD (max LOD=2.68, chromosome 17, 102cM). Additional regions suggestive of linkage for the %ucOC-wbBMC analysis were found on chromosome 2 (max LOD=2.16, 271cM) and chromosome 12 (max LOD=2.11, 67cM). Additional regions possibly influencing %ucOC-wbBMD included QTL on chromosome 1 (max LOD=2.40, 85cM) and chromosome 12 (max LOD=2.19), at the same location as the %ucOC-wbBMC peak (67cM). Suggestive evidence of linkage for the ucOC-wbBMC analysis was detected on chromosome 1 (max LOD=2.40), at the same location as the %ucOC-wbBMC peak (85cM).
DISCUSSION
We investigated the epidemiologic correlates, heritability and genome-wide linkage of serum OC concentrations among men and women belonging to very large, multi-generational African ancestry families. Osteocalcin levels were similar to previously reported values in African Americans and were lower than values reported in Caucasians [15]. Age, gender, BMI and menopausal status were the major correlates of OC; however, the majority of phenotypic variation was explained by additive genetic factors. The combination of our large, multigenerational families, large number of relative pairs and a high-density genome-wide linkage panel with ~1 cM spacing enabled us to detect several chromosomal regions harboring potential QTL for OC, including several regions not previously described.
Total OC levels in our families were similar to those reported for non-Hispanic blacks in NHANES (Tobago: 4.5μg/L vs NHANES: 3.6-4.9 μg/L), but were lower than values reported in Caucasians (3.9-5.5 μg/L)[15]. In our sample, the majority of total OC was carboxylated, with ~36% being uncarboxylated. To our knowledge, previous studies have not reported concentrations of ucOC and carbOC in African ancestry populations for comparison. However, we found a higher %ucOC (36%) than in the Caucasian Framingham cohort, which had mean values ranging from 15.6%-22.7% depending on age and gender [24]. In the Framingham sample, %ucOC was generally greater with increasing age and was also greater in women compared to men, both of which were confirmed in our African ancestry sample.
In multivariable models, women had significantly lower total and carboxylated OC than men, consistent with other reports [15, 25]. This gender difference was reversed among older individuals, due to a significant impact of menopausal status on OC levels. In postmenopausal women, OC levels were substantially higher than in men or premenopausal women, consistent with a higher rate of bone loss in these women. Unfortunately, due to sample size considerations, sex-stratified analyses were not performed. Total OC and ucOC were also lower in diabetics than in non-diabetics and ucOC was higher in hypertensive than in normotensive individuals. Carboxylated OC was associated with watching television, which may be a proxy for physical activity levels in our sample. Current smoking was associated with a higher percentage of uncarboxylated OC. A previous report by Shea et. al. found that body size and hormone replacement therapy were significantly correlated with percentage ucOC [8].
All four OC related traits had significant, high residual heritability and a very low proportion of variance explained by measured covariates in our African ancestry families. Analyses in Caucasian twins and Mexican American families have also estimated a high heritability of total OC (~0.60; 5,6), whereas another analysis of 240 Caucasian post-menopausal twins estimated heritability to be 0.38 [7]. Our results are the first to estimate the heritability of total and carboxylated forms of OC within the same sample. We found that there are strong genetic influences on total OC and ucOC, which had residual heritability estimates of 74.0% and 89.3%, respectively.
There have been few genome-wide studies of serum OC concentrations. Genome-wide association studies have become a widely used approach to identify common variation associated with disease related traits; however, these studies have explained only a very small portion of the phenotypic variance in quantitative traits to date including BMD [26]. While no genome-wide association studies (GWAS) for OC have been performed, several genome-wide linkage studies of OC have been published [3, 5, 6]. In contrast to GWAS, genome-wide linkage can identify rare-variants with larger phenotypic effects that segregate in families [27]. Although we have only recruited 7 families to date, the large multigenerational family structure and thousands of relative pairs enabled us to detect 7 chromosomal regions with suggestive evidence of univariate linkage for OC related traits. None of the linked regions contained the structural locus for OC (BGLAP), which is located on the long arm of chromosome 1 (1q25-q31). It seems likely that variation in other regions of the genome may affect circulating OC levels, such as genes related to bone mineralization and metabolism. Thus, further examination of the chromosomal regions identified herein may reveal fundamental insight into the genetic regulation of OC independent of BGLAP.
Univariate analyses only identified genomic regions suggestive of linkage with OC traits. The highest LOD score detected in univariate linkage analysis (LOD=2.83) was for total OC on chromosome 11, and this region contained no striking candidate genes for OC or bone mineral regulation. Similarly, the region on chromosome 9 (LOD=2.61; total OC) contains no strong biologic candidate. Interestingly, the region on chromosome 18 in linkage with total OC (LOD=2.31) and carbOC (LOD=2.05) contains the TNFRSF11A (RANK) gene, a key regulator of osteoclast formation and function [28, 29]. The last region suggestive of linkage with total OC was on chromosome 22 and includes the MAPK1 gene, which has been shown to be necessary for induction of the BGLAP gene [30]. We found evidence of suggestive linkage for carboxylated OC in a region on chromosome 5 (LOD 2.17). A SNP within the region (rs10078095) resides in the osteoarthritis-associated locus, GDF5-UQCC, and has been associated with human height in a genome-wide association study [31]. We also detected suggestive evidence of linkage for %ucOC for a region on chromosome 12 (LOD=2.15), which contains many potential candidate genes including the insulin-like growth factor 1 (IGF1) and transient receptor potential vanilloid 4 (TRPV4) genes, which are both involved in chondrogenesis and bone development [32, 33].
Bivariate linkage analysis of OC and whole body bone mineral may provide unique insight on the genetics of bone health. To our knowledge, there have been no previous bivariate analyses of these traits. We found that serum OC concentration was inversely correlated with BMD and BMC [2] and that part of this correlation was attributable to shared genetic influences. Using bivariate linkage analysis we were able to identify four additional peaks suggestive of linkage, including one peak on chromosome 17 that achieved genome-wide significance (LOD=3.15), that were not identified in any of our univariate analyses. This region contains 64 genes and spans 4.6MBp between 20.7-25.3MBp on chromosome 17. There are many developmental genes under this peak such as distal-less homeobox 3 and 4 (DLX3/4) and growth hormone 1 and 2 (GH1/2). However, the strongest candidate in this region appears to be the collagen type 1 alpha 1 (COL1A1) gene. Mutations in this gene have been associated with osteogenesis imperfecta [34] and osteoporosis [35]. Additionally, the Sp1 polymorphism in COL1A1 has been associated with fractures [36].
Mitchell et al. conducted a genome-wide linkage scan of total serum OC using 376 microsatellite markers spaced at ~10cM intervals in 429 Mexican American individuals comprising 10 families [3]. They identified evidence for linkage between a QTL influencing serum OC levels with markers on chromosome 16q (LOD=3.35), and suggestive evidence for linkage of OC levels with markers on chromosome 20q (LOD=2.78). The region on chromosome 16q has been replicated in 591 pedigreed baboons [6] and an additional QTL with effects on OC has been mapped to the baboon ortholog on human chromosome 6p23-21 [5]. We were unable to detect significant or suggestive evidence of linkage of serum OC concentrations with markers in these regions. Differences in the ethnic composition between our study and the report in Mexican Americans may explain the different linkage results.
A meta-analysis of 9 genome-wide linkage studies of BMD in 11,842 Caucasians identified 8 genomic regions with significant evidence of linkage to spine BMD and 6 regions with significant evidence of linkage to hip BMD [37]. None of these regions overlap directly with the regions suggestive of linkage in our univariate or bivariate analyses. Genome-wide linkage analysis of the Framingham families identified markers on chromosome 5 that were in linkage with femoral neck shaft width (LOD=3.48, men only), which overlaps with the region for carbOC in our families [38]. This region may be of particular interest for future research.
We documented an association between totalOC and ucOC with diabetes in our African ancestry families. Serum OC was lower in diabetics than in non-diabetics, which is consistent with several studies that have found a connection between OC, energy metabolism and diabetes [39]. Among healthy individuals, OC has been positively associated with glucose tolerance and beta-cell functioning [40] and inversely with diabetes prevalence [41]. Also, OC was inversely associated with BMI in our families. This observation is consistent with other reports in diabetics [40, 41] and in OC knock-out mice, which display excess visceral adiposity and insulin resistance [39]. However in mice, only uncarboxylated osteocalcin and not carboxylated osteocalcin is considered a regulator of energy metabolism. Recent reports also suggest that OC is expressed in human adipose tissues and adipoctyes and may play a role in adipogenesis [42].
The wide age range of subjects may be a potential limitation of our analysis. However, we adjusted all analyses for age and menopausal status. We also did not have information on dietary vitamin K in which may limit interpretation of our epidemiological results for carboxylated OC. However, the absence of dietary vitamin K data is unlikely to impact our linkage results. Also, we did not have data on when fractures may have occurred. Since recent fractures can increase metabolism markers, including OC, this may have influenced OC levels. However, the prevalence of fracture is low in this relatively young population, and, thus, unlikely to have impacted the linkage results.
In conclusion, serum OC concentrations appear to be largely under genetic control among African ancestry individuals. Age, gender, menopause status and other covariate effects only accounted for 3% to 8% of the inter-individual variation in serum OC concentrations. Our large, multi-generational families combined with a high-density linkage panel enabled us to detect several genomic regions that may harbor novel loci influencing serum OC concentrations and bone mineralization. Larger studies are needed to investigate these chromosomal regions in greater detail and may reveal fundamental insight into the regulation of OC and bone mineralization.
Acknowledgements
This work was supported by grants R03-AR050107 and R01-AR049747 from the NIAMS. Ms. Kuipers was supported by NHLBI grant T32-HL083825.
Footnotes
Conflict of Interest: No disclosures
REFERENCES
- 1.Lian JB, Gundberg CM. Biochemical considerations and clinical applications. Clin Orthop. 1988;226:267–291. [PubMed] [Google Scholar]
- 2.Price PA, Parthemore JG, Deftos LI, Nishimoto SK. New biochemical marker for bone metablism. Measurement by radioimmunoassay of bone gla protein in the plasma of normal subjects and patients with bone disease. J Clin Invest. 1980;66:878–883. doi: 10.1172/JCI109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell BD, Cole SA, Bauer RL, Iturria SJ, Rodriguez EA, Blangero J, MacCluer JW, Hixson JE. Genes influencing variation in serum osteocalcin concentrations are linked to markers on chromosomes 16q and 20q. J Clin Endocrinol Metab. 2000;85:1362–1366. doi: 10.1210/jcem.85.4.6571. [DOI] [PubMed] [Google Scholar]
- 4.Kelly PJ, Hopper JL, Macaskill GT, Pocock NA, Sambrook PN, Eisman JA. Genetic factors in bone turnover. J Clin Endocrinol Metab. 1991;72:808–813. doi: 10.1210/jcem-72-4-808. [DOI] [PubMed] [Google Scholar]
- 5.Havill LM, Rogers J, Cox LA, Mahaney MC. QTL with pleiotropic effects on serum levels of bone-specific alkaline phosphatase and osteocalcin maps to the baboon ortholog of human chromosome 6p23-21.3. J Bone Miner Res. 2006;21:1888–1896. doi: 10.1359/jbmr.060812. [DOI] [PubMed] [Google Scholar]
- 6.Havill LM, Cox LA, Rogers J, Mahaney MC. Cross-species replication of a serum osteocalcin quantitative trait locus on human chromosome 16q in pedigreed baboons. Calcif Tissue Int. 2005;77:205–211. doi: 10.1007/s00223-005-0056-1. [DOI] [PubMed] [Google Scholar]
- 7.Garnero P, Arden NK, Griffiths G, Delmas PD, Spector TD. Genetic influence on bone turnover in postmenopausal twins. J Clin Endocrinol Metab. 1996;81:140–146. doi: 10.1210/jcem.81.1.8550741. [DOI] [PubMed] [Google Scholar]
- 8.Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D'Agostino RB, Sr, Ordovas JM, O'Donnell CJ, Dawson-Hughes B, Vasan RS, Booth SL. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63:458–464. doi: 10.1038/sj.ejcn.1602959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuigan F, Kumar J, Ivaska KK, Obrant KJ, Gerdhem P, Akesson K. Osteocalcin gene polymorphisms influence concentration of serum osteocalcin and enhance fracture identification. J Bone Miner Res. 2010 doi: 10.1002/jbmr.32. doi: 10.1002/jmbr.32. [DOI] [PubMed] [Google Scholar]
- 10.Lorentzon M, Lorentzon R, Nordstr√∂m P. Vitamin D receptor gene polymorphism is related to bone density, circulating osteocalcin, and parathyroid hormone in healthy adolescent girls. J Bone Miner Metab. 2001;19:302–307. doi: 10.1007/s007740170014. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Morimoto S, Ishii A, Higuchi T, Ogihara T, Kakudo K. Cytosine-adenine repeat polymorphism at calcitonin gene locus associated with serum osteocalcin level in Japanese women. Cell Mol Biol. 2006;30:15–18. [PubMed] [Google Scholar]
- 12.Mullin BH, Wilson SG, Islam FM, Calautti M, Dick IM, Devine A, Prince RL. Klotho gene polymorphisms are associated with osteocalcin levels but not bone density of aged postmenopausal women. Calcif Tissue Int. 2005;77:145–151. doi: 10.1007/s00223-004-0291-x. [DOI] [PubMed] [Google Scholar]
- 13.Lau HH, Ho AY, Luk KD, Kung AW. Transforming growth factor-beta1 gene polymorphisms and bone turnover, bone mineral density and fracture risk in southern Chinese women. Calcif Tissue Int. 2004;74:516–521. doi: 10.1007/s00223-004-0163-4. [DOI] [PubMed] [Google Scholar]
- 14.Ferland G. The vitamin K-dependent proteins: an update. Nutr Rev. 1998;56:223–230. doi: 10.1111/j.1753-4887.1998.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 15.Gundberg CM, Looker AC, Nieman SD, Calvo MS. Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone. 2002;31:703–708. doi: 10.1016/s8756-3282(02)00902-x. [DOI] [PubMed] [Google Scholar]
- 16.Henry YM, Eastell R. Ethnic and gender differences in bone mineral density and bone turnover in young adults: effect of bone size. Osteoporos Int. 2000;11:512–517. doi: 10.1007/s001980070094. [DOI] [PubMed] [Google Scholar]
- 17.Miljkovic-Gacic I, Patrick AL, Kammerer CM, Bunker CH. Estimates of African, European and Native American ancestry in Afro-Caribbean men on the island of Tobago. Hum Hered. 2005;60:129–133. doi: 10.1159/000089553. [DOI] [PubMed] [Google Scholar]
- 18.Hill DD, Cauley JA, Sheu Y, Bunker CH, Patrick AL, Baker CE, Beckles GL, Wheeler VW, Zmuda JM. Correlates of bone mineral density in men of African ancestry: the Tobago bone health study. Osteoporos Int. 2008;19:227–234. doi: 10.1007/s00198-007-0450-9. [DOI] [PubMed] [Google Scholar]
- 19.Gundberg CM, Neiman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–3266. doi: 10.1210/jcem.83.9.5126. [DOI] [PubMed] [Google Scholar]
- 20.Heath SC, Snow GL, Thompson EA, Tseng C, Wijsman EM. MCMC segregation and linkage analysis. Genet Epidemiol. 1997;14:1011–1016. doi: 10.1002/(SICI)1098-2272(1997)14:6<1011::AID-GEPI75>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Kosambi D. The estimation of map distances from recombination values. Ann Eugen. 1944;12:172–175. [Google Scholar]
- 22.Almasy LBJ. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol. 1997;14:953–958. doi: 10.1002/(SICI)1098-2272(1997)14:6<953::AID-GEPI65>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.McKeown NM, Jacques PF, Gundberg CM, Peterson JW, Tucker KL, Kiel DP, Wilson PWF, Booth SL. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr. 2002;132:1329–1334. doi: 10.1093/jn/132.6.1329. [DOI] [PubMed] [Google Scholar]
- 25.Tarallo P, Henny J, Fournier B, Siest G. Plasma osteocalcin: biological variations and reference limits. Scand J Clin Lab Invest. 1990;50:649–655. doi: 10.3109/00365519009089183. [DOI] [PubMed] [Google Scholar]
- 26.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotmi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lander ES, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 28.Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–625. doi: 10.1016/j.coph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 30.Xiao G, Jiang D, Gopalakrishnan R, Fanceschi RT. Fibroblast growth factor 2 induction of the osteoclacin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002;277:36181–7. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- 31.Sanna S, Jackson AU, Nagaraja R, Willer CJ, Chen WM, Bonnycastle LL, Shen H, Timpson N, Lettre G, Usala G, Chines PS, Stringham HM, Scott LJ, Dei M, Lai S, Albai G, Crisponi L, Naitza S, Doheny KF, Pugh EW, Ben-Shlomo Y, Ebrahim S, Lawlor DA, Bergman RN, Watanabe RM, Uda M, Tuomilehto J, Coresh J, Hirschhorn JN, Shuldiner AR, Schlessinger D, Collins FS, Davey Smith G, Boerwinkle E, Cao A, Boehnke M, Abecasis GR, Mohlke KL. Common variants in the GDF5-UQCC region are associated with variation in human height. Nat Genet. 2008;40:198–203. doi: 10.1038/ng.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yakar S, Kim H, Zhao H, Toyoshima Y, Pennisi P, Gavrilova O, Leroith D. The growth hormone-insulin like growth factor axis revisited: lessons from IGF-1 and IGF-1 receptor gene targeting. Pediatr Nephrol. 2005;20:251–254. doi: 10.1007/s00467-004-1613-y. [DOI] [PubMed] [Google Scholar]
- 33.Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: the sixth sense of the musculoskeletal system? Ann N Y Sci. 2010;1192:404–409. doi: 10.1111/j.1749-6632.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, Hyland JC, Körkkö J, Prockop DJ, De Paepe A, Coucke P, Symoens S, Glorieux FH, Roughley PJ, Lund AM, Kuurila-Svahn K, Hartikka H, Cohn DH, Krakow D, Mottes M, Schwarze U, Chen D, Yang K, Kuslich C, Troendle J, Dalgleish R, Byers PH. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutation align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat Genet. 1996;14:203–205. doi: 10.1038/ng1096-203. [DOI] [PubMed] [Google Scholar]
- 36.Tran BN, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Enhancement of absolute fracture risk prognosis with genetic marker: the collagen I alpha 1 gene. Calcif Tissue Int. 2009;85:379–388. doi: 10.1007/s00223-009-9296-9. [DOI] [PubMed] [Google Scholar]
- 37.Ioannidis JP, Ng MY, Sham PC, Zintzaras E, Lewis CM, Deng HW, Econs MJ, Karasik D, Devoto M, Kammerer CM, Spector T, Andrew T, Cupples LA, Duncan EL, Foroud T, Kiel DP, Koller D, Langdahl B, Mitchell BD, Peacock M, Recker R, Shen H, Sol-Church K, Spotila LD, Uitterlinden AG, Wilson SG, Kung AW, Ralston SH. Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J Bone Miner Res. 2007;22:173–183. doi: 10.1359/jbmr.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karasik D, Dupuis J, Cho K, Cupples LA, Zhou Y, Kiel DP, Demissie S. Refined QTLs of osteoporosis-related traits by linkage analysis with genome-wide SNPs: Framingham SHARe. Bone. 2010;46:1114–1121. doi: 10.1016/j.bone.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf G. Energy regulation by the skeleton. Nutr Rev. 2008;66:229–233. doi: 10.1111/j.1753-4887.2008.00027.x. [DOI] [PubMed] [Google Scholar]
- 40.Hwang YC, Jeong IK, Ahn KJ, Chung HY. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev. 2009;25:768–772. doi: 10.1002/dmrr.1045. [DOI] [PubMed] [Google Scholar]
- 41.Lumanchi F, Camozzi V, Tombolan V, Luisetto G. Bone mineral density, osteocalcin, and bone-specific alkaline phosphatase in patients with insulin-dependent diabetes mellitus. Ann N Y Acad Sci 1173 Suppl. 2009;1:E64–67. doi: 10.1111/j.1749-6632.2009.04955.x. [DOI] [PubMed] [Google Scholar]
- 42.Foresta C, Strapazzon G, De Toni L, Gianesello L, Calcagno A, Pilon C, Plebani M, Vettor R. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-2557. doi: 10.2010/jc.2009-2557. [DOI] [PubMed] [Google Scholar]