Table 1.
Reaction parameters for C-H functionalization of indole 1 to 2.[a]
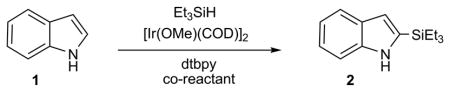
| ||||
|---|---|---|---|---|
| Entry | Et3SiH (equiv) | Co-Reactant (equiv) | Solvent | Yield [%] |
| 1 | 5 | none | Octane | 0[b] |
| 2 | 5 | none | THF | 4 |
| 3 | 5 | Cyclopentene (5) | THF | 15 |
| 4 | 5 | 2-norbornene (5) | THF | 85 |
| 5 | 5 | 2-norbornene (5) | Octane | 22 |
| 6 | 5 | 2-norbornene (5) | DME | 49 |
| 7 | 5 | 2-norbornene (5) | dioxane | 59 |
| 8 | 3 | 2-norbornene (3) | THF | 87 |
| 9 | 1.5 | 2-norbornene (1.5) | THF | 15 |
| 10 | 3 | 2-norbornene (0.5) | THF | 7 |
| 11 | 1.5 | 2-norbornene (3) | THF | 43 |
Reaction conditions: 5 mol% [Ir(OMe)(COD)]2 and 10 mol% 4,4-di-tert- butyl-2,2-bipyridine (dtbpy) at 80°C for 24 h.
At 120°C for 24 h.
