Table 2.
C-H functionalization/triethylsilylation of substituted indoles.[a]
| Entry | Indole | Adduct | Yield [%] |
|---|---|---|---|
| 1 |
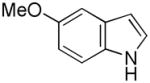 3 |
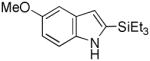 4 |
90 |
| 2 |
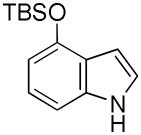 5 |
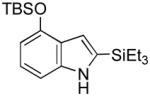 6 |
82 |
| 3 |
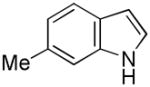 7 |
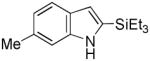 8 |
86 |
| 4 |
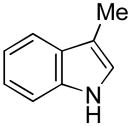 9 |
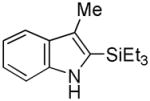 10 |
55[b] |
| 5 |
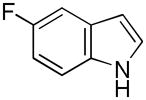 11 |
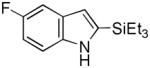 12 |
76 |
| 6 |
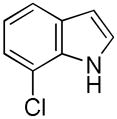 13 |
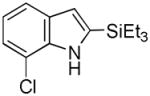 14 |
64 |
| 7 |
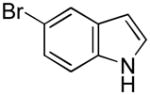 15 |
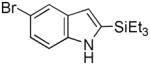 16 |
52[b] |
| 8 |
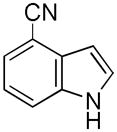 17 |
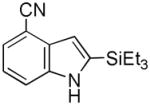 18 |
41[b] |
| 9 |
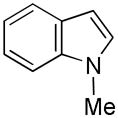 19 |
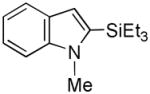 20 |
49 |
| 10 |
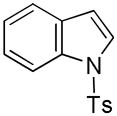 21 |
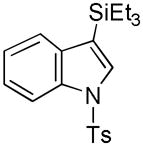 22 |
70 |
Reaction conditions: 5 mol% [Ir(OMe)(COD)]2, 10 mol% 4,4-di-tert- butyl-2,2-bipyridine (dtbpy), Et3SiH (3 equiv), and norbornene (3 equiv) in THF at 80°C for 24 h.
Same as [a], except 10 mol% [Ir(OMe)(COD)]2 and 20 mol% dtbpy for 36–48 h.
