Table 2.
Survey of cross-coupling partners.a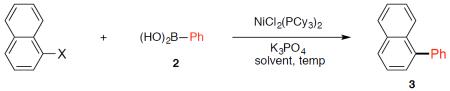
| entry | X | yield (t-amyl alcohol)b | yield (2-Me-THF)b,c |
|---|---|---|---|
| 1 | OSO2NMe2 | 100 | 100 |
| 2 | OCONEt2 | 57 | 50 |
| 3 | OPiv | 94 | 100 |
| 4 | OMs | 97 | 95 |
| 5 | OTs | 100 | 98 |
| 6 | OTf | 100 | 100 |
| 7 | Cl | 100 | 94 |
| 8 | Br | 97 | 92 |
| 9 | I | 100 | 97 |
Conditions: NiCl2(PCy3)2 complex (5 mol %), substrate (1.00 equiv), 2 (2.50 equiv), K3PO4 (4.50 equiv), hexamethylbenzene (0.10 equiv), 100 °C, 12 h.
Yield of 3 determined by 1H NMR analysis of the crude reaction mixtures using hexamethylbenzene as an internal standard.
66 °C
