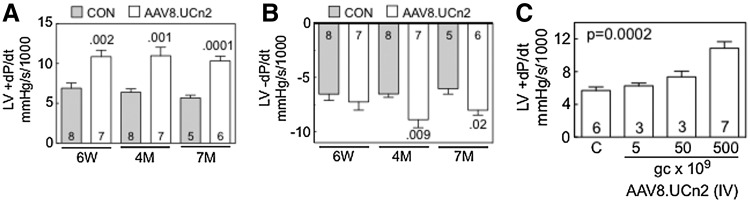
FIG. 2.
LV function in situ and AAV8.UCn2 dose–response effect. (A and B) Six weeks after AAV8.UCn2 (5×1011 GC, intravenous) or saline (CON) administration in vivo studies were performed to measure the rates of LV pressure development (LV +dP/dt) and decay (LV −dP/dt), which were used to assess LV systolic and diastolic function. AAV8.UCn2 increased LV +dP/dt at 6 weeks (p=0.002), 4 months (p=0.001), and 7 months (p=0.0001) after gene transfer, indicating that UCn2 gene transfer increases LV contractile function. Similarly, LV −dP/dt, a measure of LV diastolic function, was increased 4 months (p=0.009) and 7 months (p<0.02) after AAV8.UCn2 delivery. Studies were performed without knowledge of group identity. Values of p in (A) and (B) were determined by Student t test (unpaired, two-tailed). (C) To determine whether there was a relationship between the amount of AAV8.UCn2 delivered and its effect on LV function, LV +dP/dt was measured 6 weeks after delivery of AAV8.UCn2 in various doses: 5×109 GC (n=3); 5×1010 GC (n=3); and 5×1011 GC (n=7) versus saline-injected control mice (C) (n=6). Increased LV +dP/dt showed a dose-related effect; values of p were determined by ANOVA. 6W, 6 weeks; 4M, 4 months; 7M, 7 months; GC, genome copies; IV, intravenous; LV, left ventricle; UCn2, urocortin-2. In all graphs, data represent means±SE, and numbers in columns denote group size.